
Cervical mediastinoscopy and video-assisted mediastinoscopic lymphadenectomy for the staging of non-small cell lung cancer
Introduction
The current international guidelines for preoperative mediastinal nodal staging of non-small cell lung cancer (NSCLC) advocate obtaining the highest certainty level before lung resection to define the prognosis and decide on the most proper treatment in each case (1,2). Consequently, performing an invasive staging of the mediastinum in all cases except in those patients with small (≤3 cm) peripheral tumors with no evidence of nodal involvement on computed tomography (CT) and positron emission tomography (PET) is recommended. Invasive staging can be performed with endoscopic or surgical techniques. When both modalities are available, starting with the less invasive endoscopic techniques, such as endobronchial ultrasonographic fine-needle aspiration (EBUS-FNA), esophageal ultrasonographic FNA (EUS-FNA), or their combination (CUS), is recommended. Cervical Mediastinoscopy or more extensive surgical procedures, such as video-assisted mediastinoscopic lymphadenectomy (VAMLA), are used to validate their negative results. However, based on the latest evidence, surgical methods seem to be more accurate for those tumors with normal mediastinum staged by CT and PET.
This review summarizes the present role and performance of cervical mediastinoscopy and its technical successor, VAMLA, in the staging of NSCLC and their integration in the current preoperative staging algorithms.
Cervical mediastinoscopy
Carlens’ mediastinoscopy, more than half a century after it was first reported (3), remains the gold standard of the exploration of the superior mediastinum. Even in the era of endosonographies with the real-time puncture of peritracheal, peribronchial, and peri-esophageal lymph nodes and masses, cervical mediastinoscopy has preserved its role as a staging procedure of the mediastinum in patients with lung cancer, mesothelioma, and lung metastases (4-9).
Range of mediastinal exploration
Cervical mediastinoscopy explores the whole length of the trachea and the main bronchi. For lung cancer staging, and according to the International Association for the Study of Lung Cancer (IASLC) lymph node map (10), the range of exploration includes all nodal stations from the sternal notch bilaterally to the subcarinal and hilar nodes (Table 1).

Full table
Recommendations for mediastinal surgical staging
The intensity of this procedure depends on its indications. When cervical mediastinoscopy is indicated to confirm N3 disease, it is not necessary to continue with the exploration when an intraoperative frozen section confirms malignancy. For those cases with low suspicion of mediastinal disease on CT or PET, cervical mediastinoscopy should be systematic and thorough. It is recommended to start by exploring the contralateral paratracheal nodes to rule out or confirm N3 disease, and then to proceed with the subcarinal nodes, and finally the ipsilateral paratracheal nodes (12).
At minimum, the right and left inferior paratracheal lymph nodes and the subcarinal lymph nodes should be explored and biopsied for a clinically acceptable cervical mediastinoscopy (2). Exploration of the subaortic and para-aortic nodal stations is indicated in left lung cancers. This can be done by parasternal mediastinotomy (13,14), extended cervical mediastinoscopy (15-17), or video-assisted thoracoscopic surgery (VATS) (18).
Results
The precision of cervical mediastinoscopy depends on its thoroughness, which is itself based on the number of biopsies performed and the number of nodal stations explored (19). This accounts for the heterogeneous sensitivity and negative predictive values reported: 0.78 to 0.97 for sensitivity, and 0.91 to 0.99 for negative predictive value (1) (Table 1). The use of a video-mediastinoscope may increase sensitivity: 0.78 and 0.89 for conventional and video-assisted mediastinoscopies, respectively (20) (Table 1).
Transcervical lymphadenectomies
VAMLA, a totally endoscopic procedure, and transcervical extended mediastinal lymphadenectomy (TEMLA), an open procedure assisted by the video-mediastinoscope and the video-thoracoscope at critical points of the intervention, have emerged from the natural evolution of cervical mediastinoscopy and the improvements of video technology (21-23). The aim of both procedures is to perform a bilateral mediastinal lymphadenectomy (including lymph nodes and surrounding adipose tissue). For this reason, VAMLA and TEMLA, allow the detection of minimal nodal disease that is not identifiable on CT or PET, and thus are the ideal techniques for those tumors without suspicion of N2-3 by CT and PET. Accordingly, the following indications have been described: central tumors, cN1 tumors, left-sided tumors, bilateral synchronous lung cancer, and pre-resectional lymphadenectomy in video-assisted thoracoscopic lobectomy (11).
Range of mediastinal exploration
For VAMLA, mediastinal exploration ranges bilaterally from the superior paratracheal to the hilar nodes. For TEMLA, it ranges bilaterally from the supraclavicular to the para-esophageal nodes, including the hilar nodes (11) (Table 1). For left lung cancers, VAMLA and extended cervical mediastinoscopy are used to explore the subaortic and the para-aortic nodal stations (24-26). Those performing TEMLA include them in the exploration when the cancer is on the left lung (27,28).
Results
Sensitivity and accuracy are high, at 0.88–0.96 and 0.94–0.99, respectively (24-28) (Table 1). These results represent the best staging values reported to date for patients without suspicion of N2 by CT and PET. As we have stated previously, the high and homogenous precision reported in all series of transcervical lymphadenectomies for staging NSCLC is due to both techniques allowing the complete removal of the mediastinal nodes and the surrounding adipose tissue, instead of merely taking biopsy samples from the lymph nodes (Figure 1).
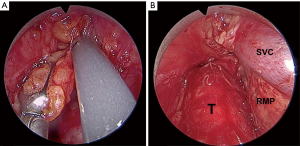
Cervical mediastinoscopy and VAMLA for staging NSCLC
Locally advanced NSCLC
The current guidelines suggest starting with an endosonographic method (EBUS-FNA, EUS-FNA, or their combination) (29-31). When these procedures are positive, this information can be enough to start a multidisciplinary treatment protocol. However, their negative results should be validated with a cervical mediastinoscopy because endosonography procedures have a high post-test probability of nodal disease (>0.10) (30-32) (Table 2). Although the ideal indication for VAMLA is in tumors with normal mediastinum identified by CT and PET, VAMLA also has been reported to be useful in patients with enlarged lymph nodes in whom frozen sections during cervical mediastinoscopy do not yield a positive result. In these cases, cervical mediastinoscopy can be converted to VAMLA (26) (Table 2).

Full table
Early stage NSCLC
European guidelines suggest avoiding invasive staging in the following situations: (I) peripheral tumors; (II) tumors ≤3 cm; (III) absence of N2 disease on CT and PET (2), a rate of unsuspected pathologic mediastinal nodal disease <10% (2,41,42). However, early stage NSCLC can have a higher risk of mediastinal involvement (33.8%) when some tumor characteristics [histological type, consolidation/tumor ratio, tumor size, maximum standardized uptake value (SUVmax value)] are combined with other clinical parameters [serum carcinoembriogenic antigen (CEA) level, patient’s age] (43,44). For this reason, in these subgroups of patients, invasive mediastinal staging can be useful (33) (Table 2). In early stage NSCLC, endosonographic methods have low sensitivity (0.17–0.41) (45-50), while in cervical mediastinoscopy, the sensitivity and negative predictive value is investigator dependent, accounting for the reported heterogeneity of 0.32 to 0.97, and 0.8 to 0.99, respectively (1,46). Transcervical lymphadenectomies, in patients without suspicion of mediastinal nodal disease by PET-CT, provide the highest clinical staging certainty, with sensitivity and negative predictive values of 0.88–0.96 and 0.94–0.99, respectively (24-28) (Table 2).
A special clinical setting of this subgroup of tumors is left lung cancer. Left lung cancers tend to spread by a lymphatic route to the right paratracheal nodal stations. If these nodal stations are not explored preoperatively, they cannot be explored from the left side by thoracotomy or by VATS with the same thoroughness as they can be explored from the right side, and they will remain unexplored. If they harbor lymph node metastases, the N3 status will be unknown, postoperative prognosis will be inaccurate, and no adjuvant treatment will be indicated. In addition, the left paratracheal nodal stations cannot be thoroughly explored from the left side unless the aortic arch is mobilized, which is a procedures that is not routinely done. Dissection of the left inferior paratracheal lymph nodes has a survival benefit and, therefore, they should not be neglected (47). From the technical point of view, these lymph nodes are more easily explored or removed by cervical mediastinoscopy or VAMLA, respectively, than by dissection from the left side by thoracotomy or VATS.
Intermediate suspicion of N2-3 disease
Tumors considered with an intermediate suspicion of N2-3 disease are the following: central tumors, cN1 tumors, and cN0 tumor size greater than 3 cm (1). The rate of unsuspected N2 disease for central or cN1 tumors ranges from 20% to 42% (26,34-36,51,52). Therefore, staging guidelines recommend this subgroup of patients undergo invasive staging of the mediastinum (1,2). For cN1 tumors by PET-CT explored by endosonography, sensitivity values of 0.38 (34) and 0.43 (52) to diagnose N2 disease have been reported. In one study, adding cervical mediastinoscopy increased sensitivity to 0.73 (34). In this clinical situation, cervical mediastinoscopy or VAMLA clearly have higher sensitivities and negative predictive values than those reported by endosonography: 0.73 and 0.92, respectively (35). Therefore, in this particular group of tumors, it seems reasonable to avoid endosonography and explore directly with cervical mediastinoscopy or VAMLA (Table 2).
For cN0 tumors more than 3 cm in greatest dimension but with high SUVmax, particularly adenocarcinomas, the unsuspected N2 rate has been reported to be between 6% and 15% (41,42). However, when patients with these tumors undergo VAMLA, the rate of unsuspected mediastinal nodal disease is as high as 22% (19% N2 and 3% N3) (26) (Table 2). Therefore, in these clinical situations, it would be prudent to confirm negative endosonography results with a surgical invasive procedure such as cervical mediastinoscopy or, better yet, VAMLA.
Conclusions
In the era of ultrasound-assisted endoscopies with real time puncture of peribronchial and periesophageal lymph nodes, cervical mediastinoscopy and VAMLA have evidence-based indications that offer the highest certainty for clinical staging of NSCLC. Therefore, they should be applied in current clinical practice according to the well-established indications.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors Marcin Zielinski and Qingdong Cao for the series “Mediastinoscopic Surgery” published in Mediastinum. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med.2019.07.01). The series “Mediastinoscopic Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-50S.
- De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:787-98. [Crossref] [PubMed]
- Carlens E. Mediastinoscopy: a method for inspection and tissue biopsy in the superior mediastinum. Dis Chest 1959;36:343-52. [Crossref] [PubMed]
- Scherpereel A, Astoul P, Baas P, et al. Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Eur Respir J 2010;35:479-95. [Crossref] [PubMed]
- Kindler HL, Ismaila N, Armato SG 3rd, et al. Treatment of malignant pleural mesothelioma: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1343-73. [Crossref] [PubMed]
- Pfannschmidt J, Klode J, Muley T, et al. Nodal involvement at the time of pulmonary metastasectomy: experiences in 245 patients. Ann Thorac Surg 2006;81:448-54. [Crossref] [PubMed]
- García-Yuste M, Cassivi S, Paleru C. Thoracic lymphatic involvement in patients having pulmonary metastasectomy: incidence and the effect on prognosis. J Thorac Oncol 2010;5:S166-9. [Crossref] [PubMed]
- Embun R, Rivas de Andrés JJ, Call S, et al. Causal model of survival after pulmonary metastasectomy of colorectal cancer: a nationwide prospective registry. Ann Thorac Surg 2016;101:1883-90. [Crossref] [PubMed]
- Call S, Rami-Porta R, Embún R, et al. Impact of inappropriate lymphadenectomy on lung metastasectomy for patients with metastatic colorectal cancer. Surg Today 2016;46:471-8. [Crossref] [PubMed]
- Rusch VW, Asamura H, Watanabe H, et al. The IASLC lung cancer staging project. A proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:568-77.
- Call S, Obiols C, Rami-Porta R. Present indications of surgical exploration of the mediastinum. J Thorac Dis 2018;10:S2601-10. [Crossref] [PubMed]
- Rami-Porta R, Call S. Invasive staging of mediastinal lymph nodes: mediastinoscopy and remediastinoscopy. Thorac Surg Clin. 2012;22:177-89. [Crossref] [PubMed]
- Stemmer EA, Calvin JW, Chandor SB, et al. Mediastinal biopsy for indeterminate pulmonary and mediastinal lesions. J Thorac Cardiovasc Surg 1965;49:405-11. [PubMed]
- McNeill TM, Chamberlain JM. Diagnostic anterior mediastinoscopy. Ann Thorac Surg 1966;2:532-9. [Crossref] [PubMed]
- Specht G. Erweiterte Mediastinoskopie. Thoraxchir Vask Chir 1965;13:401-7. [PubMed]
- Ginsberg RJ, Rice TW, Goldberg M, et al. Extended cervical mediastinoscopy. A single staging procedure for bronchogenic carcinoma of the left upper lobe. J Thorac Cardiovasc Surg 1987;94:673-8. [PubMed]
- Obiols C, Call S, Rami-Porta R, et al. Extended cervical mediastinoscopy: mature results of a clinical protocol for staging bronchogenic carcinoma of the left lung. Eur J Cardiothorac Surg 2012;41:1043-6. [Crossref] [PubMed]
- Krasna MJ. The role of thoracoscopy in the management of cancer patients. Semin Oncol 2008;35:129-33. [Crossref] [PubMed]
- Detterbeck F, Puchalski J, Rubinowitz A, et al. Classification of the thoroughness of mediastinal staging of lung cancer. Chest 2010;137:436-42. [Crossref] [PubMed]
- Zakkar M, Tan C, Hunt I. Is video mediastinoscopy a safer and more effective procedure than conventional mediastinoscopy? Interact CardioVasc Thorac Surg 2012;14:81-4. [Crossref] [PubMed]
- Rami-Porta R. Supermediastinoscopies: a step forward in lung cancer staging. J Thorac Oncol 2007;2:355-6. [Crossref]
- Hürtgen M, Friedel G, Toomes H, Fritz P. Radical video-assisted mediastinoscopic lymphadenectomy (VAMLA)— technique and first results. Eur J Cardiothorac Surg 2002;21:348-51. [Crossref] [PubMed]
- Zieliński M. Transcervical extended mediastinal lymphadenectomy: results of staging in two hundred fifty-six patients with non-small cell lung cancer. J Thorac Oncol 2007;2:370-2. [Crossref] [PubMed]
- Witte B, Wolf M, Huertgen M, et al. Video-assisted mediastinoscopic surgery: clinical feasibility and accuracy of mediastinal lymph node staging. Ann Thorac Surg 2006;82:1821-7. [Crossref] [PubMed]
- Turna A, Demirkaya A, Ozkul S, et al. Video-assisted mediastinoscopic lymphadenectomy is associated with better survival than mediastinoscopy in patients with resected non-small cell lung cancer. J Thorac Cardiovasc Surg 2013;146:774-80. [Crossref] [PubMed]
- Call S, Obiols C, Rami-Porta R, et al. Video-assisted mediastinoscopic lymphadenectomy for staging non-small cell lung cancer. Ann Thorac Surg 2016;101:1326-33. [Crossref] [PubMed]
- Zieliński M, Hauer L, Hauer J, et al. Transcervical extended mediastinal lymphadenectomy (TEMLA) for staging of non-small-cell lung cancer (NSCLC). Pneumonol Alergol Pol 2011;79:196-206. [PubMed]
- Zielinski M. Transcervical extended mediastinal lymphadenectomy (TEMLA): the standard procedure and its variations. In: Zielinski M, Rami-Porta R, eds. The transcervical approach in thoracic surgery. Heidelberg, Springer, 2014; pp. 101-16.
- Annema JT, van Meerbeeck JP, Rintoul RC, et al. Mediastinoscopy vs endosonography for mediastinal nodal staging of lung cancer: a randomized trial. JAMA 2010;304:2245-52. [Crossref] [PubMed]
- Tournoy KG, Keller S, Annema J. Mediastinal staging of lung cancer: novel concepts. Lancet Oncol 2012;13:e221-9. [Crossref] [PubMed]
- Um SW, Kim H, Jung S, et al. Endobronchial ultrasound versus mediastinoscopy for mediastinal nodal staging of non-small cell lung cancer. J Thorac Oncol 2015;10:331-7. [Crossref] [PubMed]
- Korevaar DA, Crombag L, Cohen J, et al. Added value of combined endobronchial and oesophageal endosconography for mediastinal nodal staging in lung cancer: a systematic review and meta-analysis. Lancet Respir Med 2016;4:960-8. [Crossref] [PubMed]
- Obiols C, Call S. Pros: should a patient with stage IA non-small cell lung cancer undergo invasive mediastinal staging? Transl Lung Cancer Res 2016;5:247-50. [Crossref] [PubMed]
- Dooms C, Tournoy KG, Schuurbiers O, et al. Endosonography for mediastinal nodal staging of clinical N1 non-small cell lung cancer: a prospective multicenter study. Chest 2015;147:209-15. [Crossref] [PubMed]
- Decaluwé H, Dooms C, D'Journo XB, et al. Mediastinal staging by videomediastinoscopy in clinical N1 non-small cell lung cancer: a prospective multicentre study. Eur Respir J 2017;50:1701493 [Crossref] [PubMed]
- Turna A, Melek H, Kara HV, et al. Validity of the updated European Society of Thoracic Surgeons staging guideline in lung cancer patients. J Thorac Cardiovasc Surg 2018;155:789-95. [Crossref] [PubMed]
- Yendamuri S, Battoo A, Dy G, et al. Transcervical extended mediastinal lymphadenectomy: experience from a North American Cancer Center. Ann Thorac Surg 2017;104:1644-9. [Crossref] [PubMed]
- Witte B, Messerschmidt A, Hillebrand H, et al. Combined videothoracoscopic and videomediastinoscopic approach improves radicality of minimally invasive mediastinal lymphadenectomy for early stage lung carcinoma. Eur J Cardiothorac Surg 2009;35:343-7. [Crossref] [PubMed]
- Kim HJ, Kim YH, Choi SH, et al. Video-assisted mediastinoscopic lymphadenectomy combined with minimally invasive pulmonary resection for left-sided lung cancer: feasibility and clinical impacts on surgical outcomes. Eur J Cardiothorac Surg 2016;49:308-13. [Crossref] [PubMed]
- Zieliński M, Rybak M, Solarczyk-Bombik K, et al. Uniportal transcervical video-assisted thoracoscopic surgery (VATS) approach for pulmonary lobectomy combined with transcervical extended mediastinal lymphadenectomy (TEMLA). J Thorac Dis 2017;9:878-84. [Crossref] [PubMed]
- Wang J, Welch K, Wang L, et al. Negative predictive value of positron emission tomography and computed tomography for stage T1-2N0 non-small-cell lung cancer: a meta-analysis. Clin Lung Cancer 2012;13:81-9. [Crossref] [PubMed]
- Gómez-Caro A, Boada M, Cabañas M, et al. False-negative rate after positron emission tomography/ computer tomography scan for mediastinal staging in cI stage non-small-cell lung cancer. Eur J Cardiothorac Surg 2012;42:93-100. [Crossref] [PubMed]
- Ye B, Cheng M, Li W, et al. Predictive factors for lymph node metastasis in clinical stage IA lung adenocarcinoma. Ann Thorac Surg 2014;98:217-23. [Crossref] [PubMed]
- Koike T, Koike T, Yamato Y, et al. Predictive risk factors for mediastinal lymph node metastasis in clinical stage IA non-small-cell lung cancer patients. J Thorac Oncol 2012;7:1246-51. [Crossref] [PubMed]
- Shingyoji M, Nakajima T, Yoshino M, et al. Endobronchial ultrasonography for positron emission tomography and computed tomography-negative lymph nodestaging in non-small cell lung cancer. Ann Thorac Surg 2014;98:1762-7. [Crossref] [PubMed]
- Detterbeck FC, Jantz MA, Wallace M, et al, American College of Chest Physicians. Invasive mediastinal staging of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:202S-20S.
- Zhao K, Wei S, Mei J, et al. Survival benefit of left lower paratracheal (4L) lymph node dissection for patients with left-sided non-small cell lung cancer: once neglected but of great importance. Ann Surg Oncol 2019;26:2044-52. [Crossref] [PubMed]
- Ong P, Grosu H, Eapen GA, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for systematic nodal staging of lung cancer in patients with N0 disease by computed tomography and integrated positron emission tomography-computed tomography. Ann Am Thorac Soc 2015;12:415-9. [Crossref] [PubMed]
- Vial MR, O’Connell O, Grosu H, et al. Diagnostic performance of endobronchial ultrasound-guided mediastinal lymph node sampling in early stage non-small cell lung cancer: A prospective study. Respirology 2018;23:76-81. [Crossref] [PubMed]
- Naur TM, Konge L, Clementsen P. Endobronchial ultrasound-guided transbronchial needle aspiration for staging of patients with non-small cell lung cancer without mediastinal nodal involvement at positron emission tomography-computed tomography. Respiration 2017;94:279-84. [Crossref] [PubMed]
- Lee PC, Port JL, Korst RJ, et al. Risk factors for occult mediastinal metastases in clinical stage I non-small cell lung cancer. Ann Thorac Surg 2007;84:177-81. [Crossref] [PubMed]
- Yasufuku K, Nakajima T, Waddell T, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for differentiating N0 versus N1 lung cancer. Ann Thorac Surg 2013;96:1756-60. [Crossref] [PubMed]
Cite this article as: Call S, Rami-Porta R. Cervical mediastinoscopy and video-assisted mediastinoscopic lymphadenectomy for the staging of non-small cell lung cancer. Mediastinum 2019;3:31.


