
Pediatric mediastinal germ cell tumors
Introduction
The mediastinum is the most common extragonadal primary site for germ cell tumors (GCTs) (1). Mediastinal GCTs are a rare and heterogeneous group of neoplasms. Although histologically resembling their gonadal counterparts, they differ considerably in their clinical characteristics, biological behavior and prognostic outcome. In the pediatric age group, mediastinal GCT have been found to account for 6 to 25% of all mediastinal tumors (2-7), and 3–7% of all pediatric GCTs (1,8,9).
Regardless of patient age, extragonadal GCTs, including mediastinal GCTs, arise due to arrested migration of totipotent primordial germ cells during fetal development (10). Thus, they encompass the entire range of histopathological subtypes of GCTs seen in the gonads, leading to diverse outcomes, as treatment and clinical outcomes vary according to the histology. The rarity of mediastinal GCTs has hindered their meaningful analysis, with most studies and clinical trials including them along with other extragonadal GCTs. This has led to a lack of consensus on optimal treatment strategies, and a lull in improvement in patient outcomes. Details of some studies on pediatric mediastinal GCTs are shown in Table 1 (1,4,6,9,11-13).
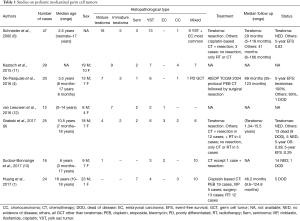
Full table
Demographics
Mediastinal GCTs display a bimodal age distribution, with one peak in infancy and the first few years of childhood, i.e., up to 5 years of age, and a second in adolescence and young adulthood, i.e., starting at 13 years of age. While teratomas and yolk sac tumors (YST) are common in the first peak, seminomas are most frequent in the second. Teratomas may also occur as congenital mediastinal masses on occasion (14). Like GCTs at other locations, mediastinal GCTs display a male preponderance (9).
Clinical presentation and diagnosis
Majority of tumors occur in the anterior mediastinum, where they may reach a large size due to the non-confining boundaries of this anatomic space (9,13). Patients may present with non-specific symptoms, or with symptoms related to mass effect: respiratory symptoms such as cough and breathlessness due to compression of the trachea, chest pain, or superior vena cava syndrome and heart failure (15-17). Occasionally, patients may present with clinical features due to hormone production by a functional tumor, such as precocious puberty (18).
The diagnosis of GCT should be considered in any pediatric patient with a mediastinal mass, and may be suspected based on the clinical assessment. The main differential diagnosis in this age group is lymphoma, which can be excluded with appropriate laboratory investigations. Like GCTs at other locations, mediastinal GCTs are associated with elevated serum levels of tumor markers, including alpha fetoprotein (AFP) and β-human chorionic gonadotropin (β-hCG). AFP is raised in YST, mixed GCTs with a YST component, and may also be elevated in some immature teratomas (9). β-hCG is markedly elevated in choriocarcinomas, or choriocarcinomatous component in a mixed GCT, and is slightly elevated in up to 20% of seminomas, usually those with syncytiotrophoblasts (19). In infants and very young children, it is important to take into consideration the age-related normal values of serum markers, as there is considerable difference from those in adults.
The next investigative procedure would be imaging studies such as computed tomography (CT) or magnetic resonance imaging (MRI), which help to identify the extent of the lesion. A definitive diagnosis can be made only on histopathological examination, and an image-guided biopsy may be performed to confirm the diagnosis in equivocal cases, e.g., in patients in whom serum markers are not within the diagnostic range. Although a rare scenario, metastases from a testicular or ovarian primary should be excluded by clinical examination and appropriate imaging prior to making a diagnosis of primary mediastinal GCT (20).
Mediastinal teratomas can occur as congenital tumors, which may grow rapidly in utero (21). This rapid growth may result in compromised venous return in the fetus, and can lead to respiratory distress in the newborn if not diagnosed antenatally. Compression of the heart and great vessels can lead to non-immune fetal hydrops (22). Prenatal ultrasound and fetal MRI aid in diagnosis and in planning the management for these tumors.
Histopathology
The histopathological features of mediastinal GCT are same as those in their testicular and ovarian counterparts. Based on their clinical and biological features, GCTs are classified into prepubertal and postpubertal types. From a treatment point of view, they are grouped as seminomas and nonseminomatous GCTs (20). The classification of mediastinal GCTs is shown in Table 2.
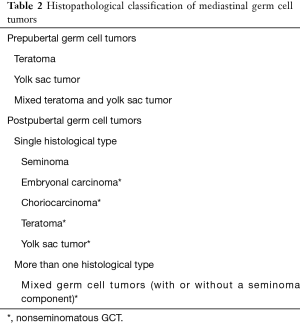
Full table
Seminomas are circumscribed, lobulated, fleshy tumors. Tumor cells are uniform, round to oval with abundant clear to pale eosinophilic cytoplasm, and central vesicular nuclei with prominent nucleoli. Thin fibrous septae separate tumor cells into lobules. These septae are infiltrated by lymphocytes and plasma cells. Clusters of epithelioid histiocytes may be present, which may also form well-defined granulomas. On occasion, extensive granulomatous reaction or fibrosis may obscure the tumor cells, making diagnosis difficult, particularly on small biopsies. Syncytiotrophoblasts may be seen interspersed between the seminoma cells, or in a perivascular location. These cells are responsible for elevation of β-hCG levels in patients with pure seminomas.
Embryonal carcinoma is a malignant GCT composed of epithelioid cells arranged in sheets, tubules and papillary structures. Tumor cells have abundant cytoplasm, and large vesicular nuclei with prominent macronucleoli. Brisk mitotic activity, apoptosis and necrosis are frequently present.
YST recapitulates the morphological features of the embryonal yolk sac and allantois, displaying a wide range of architectural patterns including microcystic, macrocystic, reticular, glandular, solid, pseudopapillary, polyvascular vitelline, hepatoid, etc. These patterns do not have an impact on the biological behavior of YSTs. Schiller-Duval bodies, typically seen in YSTs, consist of a central vessel lined by an inner layer of tumor cells that are separated from an outer layer by a clear space.
Teratomas are composed of elements from all three germ cell layers viz. ectoderm, mesoderm and endoderm. They are classified as mature when comprised of well-differentiated tissues, such as skin, hair, teeth, gastrointestinal mucosa, pancreatic tissue, skeletal muscle, bone, cartilage, glial tissue, etc., and as immature when they contain immature neuroepithelium or other undifferentiated elements. Pure immature teratomas are rare; most immature teratomas are associated with another malignant GCT, i.e., are mixed GCTs. Somatic non-germ cell malignancies may arise within a teratoma when any one of the various components undergoes malignant transformation. Some of the somatic malignancies reported in pediatric mediastinal teratomas include rhabdomyosarcoma and angiosarcoma (23-27). These malignant components usually do not respond to chemotherapy.
Choriocarcinoma is a highly malignant neoplasm composed of syncytiotrophoblasts and cytotrophoblasts, accompanied by foci of hemorrhage and necrosis. There is considerable nuclear atypia, and frequent mitoses, including atypical forms.
Following chemotherapy, excised GCTs show areas of necrosis, lympho-histiocytic infiltration, and fibrosis. Viable tumor may not be identifiable, making it difficult to document all the components present within the tumor. On relapse, new GCT components may be present, which were not present in the primary tumor.
Immunohistochemistry (IHC)
In the era of neoadjuvant therapy, the diagnosis of GCTs is made on fine needle aspirates or core needle biopsies, accompanied by serum tumor marker levels. In this scenario, due to morphological overlap between the histological types of GCTs, or small proportions of each type being present, IHC is often necessary to document each of the GCT components present within a tumor. IHC also helps to distinguish GCTs from their differential diagnoses like lymphoma, thymic carcinoma, Ewing sarcoma, rhabdomyosarcoma, etc. The immunohistochemical markers (20) used in diagnosis of GCTs are summarized in Table 3. An important point to note, especially when evaluating biopsies from metastatic sites is that GCTs, particularly YST, can show immunopositivity for cytokeratins used in panels for metastatic carcinomas, such as focal CK7 and CK20 staining, and diffuse CK19 positivity, similar to adenocarcinomas.

Full table
Staging
Pediatric GCTs use post-surgical staging systems different from those in adults. The Children’s Oncology Group (COG) staging system is used most frequently for risk assessment in pediatric patients. The post-surgical staging protocol is as follows:
- Stage I: complete resection with negative margins;
- Stage II: microscopic residual tumor or persistently raised tumor markers, with negative lymph nodes;
- Stage III: lymph node involvement, gross residual disease, or biopsy only;
- Stage IV: distant metastases.
For patients with extragonadal GCT, COG stage I/II are considered as intermediate risk, while COG stage III/IV are considered as high risk.
A combination of age, tumor site and stage has been used in risk stratification systems. The Malignant Germ Cell International Collaborative (MaGIC) initiative, an international collaborative effort set up in 2009, aimed to establish a risk stratification system for pediatric GCTs which could be used internationally in the setting of clinical trials (28). For extragonadal GCT, according to this system, low risk was defined as any age and COG stage I; standard risk 1 was defined as age <11 years and COG stage II–IV; standard risk 2 as age ≥11 and COG stage II; poor risk included patients aged ≥11 years and COG stage III–IV. The objective of this stratification system was to identify patients with worse prognosis in whom first-line therapy could be intensified, and those with a potentially excellent outcome in whom therapy could be de-intensified.
Biology
Congenital chromosomal abnormalities such as Klinefelter syndrome are associated with increased risk for development of mediastinal GCTs (19,20). Williams et al. performed array genotyping in a large cohort of 433 male pediatric and adolescent patients and identified Klinefelter syndrome in 13 patients, 9 (69%) of which were in the mediastinum, and accounted for 31% of all mediastinal GCTs included in the study (29). A role for genetic risk factors such as isochromosome 12p for development of GCT was first identified in adult testicular GCTs, and was later found to be present in mediastinal post-pubertal GCTs as well.
A few studies have shown that GCTs in all age groups have shared genetic risk factors, which are present in gonadal as well as extragonadal GCTs (30). Marcotte et al., in a case-parent triad study of pediatric and adolescent gonadal and extragonadal GCTs, reported that variants in BAK1, SPRY4, and GAB2 genes are associated with increased risk for developing GCT. However, they did not specify the location of extragonadal tumors apart from intracranial extragonadal GCT (30).
With the availability of high throughput technologies, the molecular landscape of GCTs at various locations is being explored extensively. Whole exome sequencing studies have identified mutations in MAPK and PI3K pathways, i.e., in KIT, RAS, and mTOR, among other genes (31,32) in gonadal and extragonadal GCTs, suggesting that PI3K/AKT/mTOR inhibitors may serve as novel targeted therapies for refractory GCTs. A significant enrichment in the genes in the Jumonji domain-containing (JMJD) has been reported in GCTs of the central nervous system. JMJD consists of a group of histone deacetylases which play a role in cellular processes such as transcriptional regulation and chromatin remodeling, has been found to be necessary for development of germ cells in mice, and interacts with the androgen receptor in humans (31). This remains to be evaluated in extragonadal GCTs other than those in intracranial location.
Feldman et al., in their study on 70 GCTs, including 9 mediastinal GCTs, reported that chemotherapy resistant tumors harbored more frequent mutations in AKT1 and PIK3CA genes as compared to chemo-sensitive tumors, and frequency of mutations was higher in mediastinal GCTs as compared to other locations (33). There are only occasional studies describing the genetic landscape of mediastinal GCT per se. Nappi et al., in an abstract, reported the results of targeted sequencing in 11 mediastinal GCTs, including pediatric and adult patients, in which they identified 12p amplification in 5 tumors, and mutations in KIT (2 tumors), KRAS (1 tumor), TP53 (2 tumors) and PTEN (1 tumor) (34). TP53 mutation was associated with aggressive disease and poor outcome. Thus, although the number of cases analyzed was small, they could generate sufficient data to serve as a leading point for multicenter collaborative studies with larger number of cases.
Altered gene expression due to changes in DNA methylation is postulated to play a role in pathogenesis of cancers. Global analyses of methylation profiles of various neoplasms have shown that methylation is a widespread event in cancer development. Apart from genetic alterations, epigenetic mechanisms also probably contribute to the development of GCTs. With this background, Jeyapalan et al. examined the methylation status of pediatric GCTs, including seminomas and YSTs. They found that YSTs were hypermethylated at many gene regulatory loci, and displayed a methylator phenotype different from that seen in seen in other tumor types (35). Many of the genes silenced by hypermethylation were known tumor suppressor genes, which could be responsible for the more aggressive behavior of YST as compared to seminomas. The methylator phenotype was associated with increased expression of the DNA methyl transferase DNMT3B, suggesting that this might be the mechanism for the methylator phenotype in these neoplasms.
Treatment
The treatment of mediastinal GCTs aims at complete remission and is planned as per the histological type of tumor and stage of the disease. Available treatment options include surgery, chemotherapy and radiotherapy, and a combination of these modalities.
Chemotherapy
Aggressive cisplatin-based multidrug chemotherapy followed by complete resection of residual tumor is the mainstay in the management of malignant mediastinal GCTs. Results of clinical trials have shown that pediatric mediastinal malignant GCTs that received 3–4 cycles of chemotherapy had a higher rate of complete resection as compared to those that underwent upfront surgical resection (6). Preoperative chemotherapy helps to reduce the tumor bulk, making it amenable to complete resection, especially if there is infiltration into adjacent organs. Reduced tumor vascularity and friability following chemotherapy also help prevent capsule violation during surgery (13). The various chemotherapy protocols for extragonadal/ mediastinal GCT are given in Table 4 (36-45). BEP is the most common regimen administered, with cisplatin 20 mg/m2, etoposide 100 mg/m2 and bleomycin 15 mg/m2 (1). In total, 20–30% of patients demonstrate cisplatin resistance (33). Substitution of cisplatin by carboplatin in some clinical trials did not result in improved outcome (46,47). However, Shaikh et al., in their systematic review of malignant GCT treatment, concluded that carboplatin could be utilized in young children with low to intermediate risk features, and cisplatin in older children or those with high risk features (46).
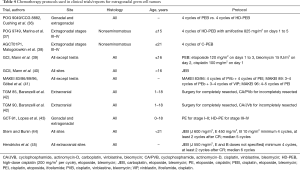
Full table
With the advent of multi-agent chemotherapy, the outcomes of patients with localized disease showed significant improvement. However, this was not paralleled by improved outcomes for advanced disease. Although the role of high-dose chemotherapy has mainly been studied in adults, a few studies have documented its effects in pediatric GCTs. The Pediatric Oncology Group and the Children’s Cancer Group conducted a randomized trial to determine effectiveness of high dose cisplatin-based chemotherapy in pediatric patients with high risk disease. The study included 39 mediastinal GCTs. While the event-free survival was significantly higher in patients who received the high-dose regimen, toxicities were significant, precluding the use of this regimen (36).
Subsequently, high dose chemotherapy (HDC) with hematopoietic progenitor cell support (HPCS) in the form of peripheral blood progenitor cells has been investigated as an option for salvage therapy in cisplatin-resistant gonadal and extragonadal GCTs. De Giorgi et al. reported results of salvage HDC in 23 children with relapsed extragonadal GCT. Various HDC protocols were administered, the most common being Carboplatin, etoposide and cyclophosphamide; HPCS included peripheral blood progenitor cells, autologous bone marrow transplantation, or both. Complete remission was achieved in 70% of patients. Of the two patients with mediastinal tumors, one achieved complete remission with no evidence of disease at 5 years, while the other had progressive disease (48). More recently, Ocal et al. compared the outcome of mediastinal GCT patients after hematopoietic stem cell transplantation with non-mediastinal extragonadal GCTs. They found that mediastinal GCT patients had better 5-year survival rates than non-mediastinal GCTs (64.9% vs. 34.7%). However, the authors did not specify the number of patients that were from the pediatric age group (49). Thus, HDC with HPCS as salvage treatment can induce remission in children with extragonadal extracranial GCTs. However, prospective randomized trials are necessary to confirm this finding.
Growing teratoma syndrome
There is a differential response of the various histological types of GCT to chemotherapy, with malignant elements like YST, embryonal carcinoma and choriocarcinoma being highly sensitive, and teratomas being relatively insensitive. Mixed GCTs consist of variable proportions of each of these components. In rare mixed GCT patients, chemotherapy is followed by a paradoxical increase in tumor size despite normalization of previously elevated tumor markers. In the mediastinum, tumor enlargement can lead to compression of vital organs, resulting in symptoms due to cardiopulmonary distress such as chest pain and breathlessness (50). This is known as Growing teratoma syndrome. According to the criteria proposed by Logothetis et al., on surgical resection, the specimen shows only a mature teratoma component (51). However, in mediastinal mixed GCTs, patients with growing teratoma syndrome have been found to have predominantly mature teratoma accompanied by small foci of malignant GCT or somatic malignancy within a teratoma (50,52). Growing teratoma syndrome is managed by stopping chemotherapy and referring the patient for surgery, which is the gold standard (53). In inoperable cases, bevacizumab has been found to be of some benefit in limiting the size of the growing teratoma (54).
Surgery
Surgery is the mainstay of treatment for mature teratomas. Surgical resection is also performed in non-teratomatous GCTs after neo-adjuvant chemotherapy to achieve complete remission. As most tumors are situated in the anterior mediastinum, anterolateral thoracotomy, posterolateral thoracotomy or median sternotomy approaches can be used to reach the tumor. Tumors often encase the major vessels. They are also frequently adherent to pleura and pericardium, which may be resected along with the tumor if required. Partial or complete thymectomy may also be required (6). In all cases, complete excision is the aim of the surgery, and the plan for surgery is primarily based on the type of GCT.
Mature teratomas of the mediastinum are best dealt with by surgical excision, as they can be completely excised and render remission. Surgical excision should be complete and spill-proof to avoid seeding in the mediastinum and recurrence later in life. In case of congenital mediastinal teratomas with features of non-immune hydrops or respiratory distress, ex utero intrapartum treatment (EXIT) has been reported in occasional cases (22). In this procedure, a multidisciplinary team conducts the surgery under maternal general anesthesia. A cesarean section is performed to permit exposure of the fetal chest by delivering the head, neck, arms and upper trunk, following which the airway can be evaluated and secured. A large mediastinal tumor may then be excised via median sternotomy approach (22).
Studies reporting outcome specifically for mediastinal subsets of children and adolescents with malignant GCTs treated with platinum-based regimens suggest that although this site is considered less favorable, good event-free survival rates can be achieved. Surgery is therefore regarded as adjuvant therapy for resection of residual tumors in patients who respond to chemotherapy. In patients that do not respond to first-line chemotherapy, surgery may be attempted for tumors that are amenable to complete resection, in view of poor response to second line chemotherapy and limited therapeutic options (55). Surgical resection of the tumor, either at onset or as post-induction surgery, leads to improvement in overall survival. It not only helps to remove tumor tissue that is resistant to chemotherapy, but histological examination of the tumor helps assess pathological response to chemotherapy and plan further management.
Radiotherapy
Despite the considerable advances made in the field of radiation oncology in recent years, there is no consensus on whether combination of radiotherapy with chemotherapy and/or surgery is an effective treatment option for mediastinal GCTs, and only occasional studies have evaluated the benefit of radiation in patients with malignant mediastinal GCTs.
Wang et al. evaluated the role of radiotherapy at a median dose of 52 Gy in a study on 61 patients with primary malignant mediastinal non-seminomatous GCTs, and found that patients receiving radiation had improved five-year overall and progression-free survival rates as compared to those who not receiving it. They concluded that radiotherapy could be a wise option for treatment of tumors refractory to platinum-based chemotherapy, to reduce local recurrence and improve survival of malignant mediastinal non-seminomatous GCT patients (56).
Huang et al. administered radiation to 12 patients at a median dose of 36 Gy for seminomatous (7 patients) and 50 Gy for non-seminomatous (5 patients) mediastinal GCTs, six following chemotherapy and six following chemotherapy and surgery. They found that none of the patients that were irradiated developed local recurrence. The 7 seminomatous GCT patients had sustained complete response (5 year DFS 100%), while 1 of the 5 non-seminomatous GCT patients relapsed with distant metastasis (5-year DFS 66.7%). They concluded that in patients with residual tumor following neoadjuvant chemotherapy and surgery, salvage radiation is a viable therapeutic option, and definitive radiotherapy may be effective in management of patients with incomplete response after chemotherapy who do not undergo surgical resection (1).
Thus, it appears that, as vital mediastinal structures can tolerate the radiation doses used, radiotherapy may be an effective alternative to surgical resection for tumors refractory to chemotherapy, as well as for inoperable tumors, and local relapses in patients previously treated with chemotherapy.
Outcome
Since its advent in the 1970s, the use of platinum-based chemotherapy regimens has led to improved survival rates for children with GCTs. However, due to the marked heterogeneity, these good outcomes are not evenly distributed across patient age, tumor location, and histological tumor type. Mediastinal GCTs have been found to have worse prognosis as compared to gonadal GCTs as well as other extragonadal GCTs (42,57,58). Their proximity to vital structures, which they often infiltrate, may be responsible for this to some extent (6). Five-year overall survival rates range from 40–60% (1,9,58,59). Mature teratomas have an excellent prognosis following complete excision, while immature teratomas have a fair outcome after surgery followed by chemotherapy.
An age ≥11 years, advanced stage disease, incomplete excision (9), absence of response and resistance to first-line chemotherapy, associated hematologic malignancies, distant metastases, and somatic-type malignancies arising in GCTs have been found to be significantly associated with worse long-term disease-free survival (19,47). Distant metastases have been reported in up to 47% of patients (9,40,58,60,61).
Mediastinal GCTs have been found to be associated with the development of hematological neoplasms, particularly myeloid neoplasms (10,60-67). Among myeloid neoplasms, those of megakaryocytic lineage are most frequent (68). This association is seen predominantly in non-seminomatous GCT, particularly in YST, and in adolescents or young adult males (68,69). These hematological malignancies may occur synchronously or subsequently, usually within a year (10). They are believed to arise from hematopoietic precursor cells within the YST component. Initially thought to be therapy-related, they were subsequently found to harbor cytogenetic abnormalities seen in GCTs, e.g., isochromosome 12p, indicating a common clonal origin. The incidence of hematological malignancies in mediastinal GCT patients has been reported to be as high as 60% (10,61); however, as this data is mostly derived from small series, it is likely to be an overrepresentation. Myeloid leukemia in GCT patients usually has a dismal prognosis, with allogeneic bone marrow transplantation being the only curative treatment option (10,68), and majority of patients not surviving beyond 5 years (68).
Challenges and insights into future development
Diagnosis of mediastinal GCT requires a multipronged approach, and is followed by multidisciplinary treatment including chemotherapy followed by surgery, with or without radiotherapy. In view of good response rates to current management protocols, the focus needs to be shifted to identifying patients in whom treatment regimens can be downscaled with the aim of decreasing long term morbidity and improving quality of life in low risk patient groups, while improving survival rates in poor risk patient subsets. In this scenario, better understanding of the molecular pathogenesis of these tumors may lead to identification of novel biomarkers and therapeutic targets, as well as improved disease segmentation and risk stratification, thus helping to avoid the toxicity and morbidity associated with current one-fits-all treatment strategies. Due to the rarity of mediastinal GCTs, multi-institutional collaborations across continents are necessary to generate meaningful data, and are the face of future developments in this arena.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor Deepali Jain for the series “Pediatric Mediastinal Tumors” published in Mediastinum. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med.2019.07.02). The series “Pediatric Mediastinal Tumors” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Huang J, Tan Y, Zhen Z, et al. Role of post-chemotherapy radiation in the management of children and adolescents with primary advanced malignant mediastinal germ cell tumors. PLoS One 2017;12:e0183219 [Crossref] [PubMed]
- Billmire DF. Germ cell, mesenchymal, and thymic tumors of the mediastinum. Semin Pediatr Surg 1999;8:85-91. [Crossref] [PubMed]
- Mullen B, Richardon JD. Primary anterior mediastinal tumors in children and adults. Ann Thorac Surg 1986;42:338-45. [Crossref] [PubMed]
- De Pasquale MD, Crocoli A, Conte M, et al. Mediastinal Germ Cell Tumors in Pediatric Patients: A Report From the Italian Association of Pediatric Hematology and Oncology. Pediatr Blood Cancer 2016;63:808-12. [Crossref] [PubMed]
- Moran CA, Suster S. Primary germ cell tumors of the mediastinum, analysis of 322 cases with special emphasis on teratomatous lesions and a proposal for histopathologic classification and clinical staging. Cancer 1997;80:681-90. [Crossref] [PubMed]
- Schneider DT, Calaminus G, Reinhard H, et al. Primary mediastinal germ cell tumors in children and adolescents: results of the German cooperative protocols MAKEI 83/86, 89, and 96. J Clin Oncol 2000;18:832-9. [Crossref] [PubMed]
- Sarkaria IS, Bains MS, Sood S, et al. Resection of primary mediastinal non-seminomatous germ cell tumors: a 28-year experience at memorial sloan-kettering cancer center. J Thorac Oncol 2011;6:1236-41. [Crossref] [PubMed]
- Schneider DT, Calaminus G, Koch S, et al. Epidemiologic analysis of 1442 children and adolescents registered in the German germ cell tumor protocols. Pediatr Blood Cancer 2004;42:169-75. [Crossref] [PubMed]
- Grabski DF, Pappo AS, Krasin MJ, et al. Long-term outcomes of pediatric and adolescent mediastinal germ cell tumors: a single pediatric oncology institutional experience. Pediatr Surg Int 2017;33:235-44. [Crossref] [PubMed]
- Sowithayasakul P, Sinlapamongkolkul P, Treetipsatit J, et al. Hematologic Malignancies Associated With Mediastinal Germ Cell Tumors: 10 Years' Experience at Thailand's National Pediatric Tertiary Referral Center. J Pediatr Hematol Oncol 2018;40:450-5. [Crossref] [PubMed]
- Kaatsch P, Häfner C, Calaminus G, et al. Pediatric germ cell tumors from 1987 to 2011: incidence rates, time trends, and survival. Pediatrics 2015;135:e136-43. [Crossref] [PubMed]
- van Leeuwen MT, Gurney H, Turner JJ, et al. Patterns and trends in the incidence of paediatric and adult germ cell tumours in Australia, 1982-2011. Cancer Epidemiol 2016;43:15-21. [Crossref] [PubMed]
- Sudour-Bonnange H, Faure-Conter C, Martelli H, et al. Primary mediastinal and retroperitoneal malignant germ cell tumors in children and adolescents: Results of the TGM95 trial, a study of the French Society of Pediatric Oncology (Société Française des Cancers de l'Enfant). Pediatr Blood Cancer 2017; [Crossref] [PubMed]
- Bekker A, Goussard P, Gie R, et al. Congenital anterior mediastinal teratoma causing severe airway compression in a neonate. BMJ Case Rep 2013; [Crossref] [PubMed]
- Rescorla FJ. Pediatric germ cell tumors. Semin Surg Oncol 1999;16:144-58. [Crossref] [PubMed]
- Billmire D, Vinocur C, Rescorla FJ, et al. Malignant mediastinal germ cell tumors: an intergroup study. J Pediatr Surg 2001;36:18-24. [Crossref] [PubMed]
- Chetaille B, Massard G, Falcoz PE. Les tumeurs germinales du mediastin: anatomopathologie, classification, tératomes et tumeurs malignes. Revue De Pneumol Clin 2010;66:63-70. [Crossref]
- Bravo-Balado A, Torres Castellanos L, Carrillo Rodríguez A, et al. Primary Mediastinal Pure Seminomatous Germ Cell Tumor (Germinoma) as a Rare Cause of Precocious Puberty in a 9-Year-Old Patient. Urology 2017;110:216-9. [Crossref] [PubMed]
- Shaikh F, Murray MJ, Amatruda JF, et al. Paediatric extracranial germ-cell tumours. Lancet Oncol 2016;17:e149-62. [Crossref] [PubMed]
- Moreira AL, Chan JKC, Looijenga LHJ, et al. Germ cell tumours of the mediastinum. In: Travis WD, Brambilla E, Burke AP, et al. editors. WHO classification of tumours of the lung, pleura, thymus and heart (4th edition), Lyon: IARC, 2015:244-66.
- Peiró JL, Sbragia L, Scorletti F, et al. Management of fetal teratomas. Pediatr Surg Int 2016;32:635-47. [Crossref] [PubMed]
- Agarwal A, Rosenkranz E, Yasin S, et al. EXIT procedure for fetal mediastinal teratoma with large pericardial effusion: a case report with review of literature. J Matern Fetal Neonatal Med 2018;31:1099-103. [Crossref] [PubMed]
- Faure Conter C, Fresneau B, Thebaud E, et al. Two Tumors in 1: What Should be the Therapeutic Target? Pediatric Germ Cell Tumor With Somatic Malignant Transformation. J Pediatr Hematol Oncol 2017;39:388-94. [Crossref] [PubMed]
- Bakshi N, Bishnoi S, Rao S. Vasoformative Lesions in Mediastinal Mixed Germ Cell Tumors: an Interesting Account of Two Cases Spanning the Benign to Malignant Spectrum. Indian J Surg Oncol 2018;9:624-8. [Crossref] [PubMed]
- Moran CA, Suster S. Germ-cell tumors of the mediastinum. Adv Anat Pathol 1998;5:1-15. [Crossref] [PubMed]
- Manivel C, Wick M R, Abenoza P, et al. The occurrence of sarcomatous components in primary mediastinal germ cell tumors. Am J Surg Pathol 1986;10:711-7. [Crossref] [PubMed]
- Wick MR, Perlman EJ, Strobel P, et al. Germ cell tumours with somatic-type malignancy. In: Travis WD, Brambilla E, Muller-Hermelink HK, et al. editors. Pathology and genetics, tumours of the lung, pleura, thymus and heart. World Health Organization classification of tumours. Lyon, France: IARC Press, 2004:188-95.
- Frazier AL, Hale JP, Rodriguez-Galindo C, et al. Revised risk classification for pediatric extracranial germ cell tumors based on 25 years of clinical trial data from the United Kingdom and United States. J Clin Oncol 2015;33:195-201. [Crossref] [PubMed]
- Williams LA, Pankratz N, Lane J, et al. Klinefelter syndrome in males with germ cell tumors: A report from the Children's Oncology Group. Cancer 2018;124:3900-8. [Crossref] [PubMed]
- Marcotte EL, Pankratz N, Amatruda JF, et al. Variants in BAK1, SPRY4, and GAB2 are associated with pediatric germ cell tumors: a report from the Children’s Oncology Group. Genes Chromosomes Cancer 2017;56:548-58. [Crossref] [PubMed]
- Wang L, Yamaguchi S, Burstein MD, et al. Novel somatic and germline mutations in intracranial germ cell tumours. Nature 2014;511:241-5. [Crossref] [PubMed]
- Ichimura K, Fukushima S, Totoki Y, et al. Recurrent neomorphic mutations of MTOR in central nervous system and testicular germ cell tumors may be targeted for therapy. Acta Neuropathol 2016;131:889-901. [Crossref] [PubMed]
- Feldman DR, Iyer G, Van Alstine L, et al. Presence of somatic mutations within PIK3CA, AKT, RAS, and FGFR3 but not BRAF in cisplatin- resistant germ cell tumors. Clin Cancer Res 2014;20:3712-20. [Crossref] [PubMed]
- Nappi L, Annala M, Vandekerkhove G, et al. Molecular dissection of primary mediastinal germ cell tumors. J Clin Oncol 2017;35:417. [Crossref]
- Jeyapalan JN, Noor DA, Lee SH, et al. Methylator phenotype of malignant germ cell tumours in children identifies strong candidates for chemotherapy resistance. Br J Cancer 2011;105:575-85. [Crossref] [PubMed]
- Cushing B, Giller R, Cullen JW, et al. Randomized comparison of combination chemotherapy with etoposide, bleomycin, and either high-dose or standard-dose cisplatin in children and adolescents with high-risk malignant germ cell tumors: a pediatric intergroup study--Pediatric Oncology Group 9049 and Children's Cancer Group 8882. J Clin Oncol 2004;22:2691-700. [Crossref] [PubMed]
- Marina N, Chang KW, Malogolowkin M, et al. Amifostine does not protect against the ototoxicity of high-dose cisplatin combined with etoposide and bleomycin in pediatric germ-cell tumours: a Children’s Oncology Group study. Cancer 2005;104:841-7. [Crossref] [PubMed]
- Malogolowkin MH, Krailo M, Marina N, et al. Pilot study of cisplatin, etoposide, bleomycin, and escalating dose cyclophosphamide therapy for children with high risk germ cell tumors: a report of the children’s oncology group (COG). Pediatr Blood Cancer 2013;60:1602-5. [Crossref] [PubMed]
- Mann JR, Pearson D, Barrett A, et al. Results of the United Kingdom Children’s Cancer Study Group’s malignant germ cell tumor studies. Cancer 1989;63:1657-67. [Crossref] [PubMed]
- Mann JR, Raafat F, Robinson K, et al. The United Kingdom Children’s Cancer Study Group’s second germ cell tumor study: carboplatin, etoposide, and bleomycin are eFFective treatment for children with malignant extracranial germ cell tumors, with acceptable toxicity. J Clin Oncol 2000;18:3809-18. [Crossref] [PubMed]
- Göbel U, Schneider DT, Calaminus G, et al. Germ-cell tumors in childhood and adolescence. GPOH MAKEI and the MAHO study groups. Ann Oncol 2000;11:263-71. [Crossref] [PubMed]
- Baranzelli MC, Kramar A, Bouffet E, et al. Prognostic factors in children with localized malignant nonseminomatous germ cell tumors. J Clin Oncol 1999;17:1212-8. [Crossref] [PubMed]
- Lopes LF, Macedo CRP, Pontes EM, et al. Cisplatin and etoposide in childhood germ cell tumor: Brazilian pediatric oncology society protocol GCT-91. J Clin Oncol 2009;27:1297-303. [Crossref] [PubMed]
- Stern JW, Bunin N. Prospective study of carboplatin-based chemotherapy for pediatric germ cell tumors. Med Pediatr Oncol 2002;39:163-7. [Crossref] [PubMed]
- Hendricks M, Davidson A, Pillay K, et al. Carboplatin-based chemotherapy and surgery: A cost effective treatment strategy for malignant extracranial germ cell tumours in the developing world. Pediatr Blood Cancer 2011;57:172-4. [Crossref] [PubMed]
- Shaikh F, Nathan PC, Hale J, et al. Is there a role for carboplatin in the treatment of malignant germ cell tumors? A systematic review of adult and pediatric trials. Pediatr Blood Cancer 2013;60:587-92. [Crossref] [PubMed]
- Bokemeyer C, Nichols CR, Droz JP, et al. Extragonadal germ cell tumors of the mediastinum and retroperitoneum: results from an international analysis. J Clin Oncol 2002;20:1864-73. [Crossref] [PubMed]
- De Giorgi U, Rosti G, Slavin S, et al. European Group for Blood and Marrow Transplantation Solid Tumours and Paediatric Disease Working Parties. Salvage high-dose chemotherapy for children with extragonadal germ-cell tumours. Br J Cancer 2005;93:412-7. [Crossref] [PubMed]
- Ocal N, Yildiz B, Karadurmus N, et al. Comparison of the clinical features and hematopoietic stem cell transplantation outcomes of mediastinal malignant germ cell tumors with nonmediastinal extragonadal placements. Onco Targets Ther 2016;9:7445-50. [Crossref] [PubMed]
- Kesler KA, Patel JB, Kruter LE, et al. The "growing teratoma syndrome" in primary mediastinal nonseminomatous germ cell tumors: criteria based on current practice. J Thorac Cardiovasc Surg 2012;144:438-43. [Crossref] [PubMed]
- Logothetis CJ, Sammuels ML, Trindade A, et al. The growing teratoma syndrome. Cancer 1982;50:1629-35. [Crossref] [PubMed]
- Iyoda A, Hiroshima K, Yusa T, et al. The primary mediastinal growing teratoma syndrome. Anticancer Res 2000;20:3723-6. [PubMed]
- Sachdeva AK, Penumadu P, Kohli P, et al. Growing teratoma syndrome in primary mediastinal germ cell tumor: our experience. Asian Cardiovasc Thorac Ann 2019;27:98-104. [Crossref] [PubMed]
- Mego M, Recková M, Sycova-Mila Z, et al. Bevacizumab in a growing teratoma syndrome. Case report. Ann Oncol 2007;18:962-3. [Crossref] [PubMed]
- Vuky J, Bains M, Bacik J, et al. Role of postchemotherapy adjunctive surgery in the management of patients with nonseminoma arising from the mediastinum. J Clin Oncol 2001;19:682-8. [Crossref] [PubMed]
- Wang J, Bi N, Wang X, et al. Role of radiotherapy in treating patients with primary malignant mediastinal non-seminomatous germ cell tumor: A 21-year experience at a single institution. Thorac Cancer 2015;6:399-406. [Crossref] [PubMed]
- Mann JR, Raafat F, Robinson K, et al. UKCCSG’s germ cell tumour (GCT) studies: Improving outcome for children with malignant extracranial non-gonadal tumours—Carboplatin, etoposide, and bleomycin are effective and less toxic than previous regimens. United Kingdom Children’s Cancer Study Group. Med Pediatr Oncol 1998;30:217-27. [Crossref] [PubMed]
- . International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J Clin Oncol 1997;15:594-603. [Crossref] [PubMed]
- Liu TZ, Zhang DS, Liang Y, et al. Treatment strategies and prognostic factors of patients with primary germ cell tumors in the mediastinum. J Cancer Res Clin Oncol 2011;137:1607-12. [Crossref] [PubMed]
- DeMent SH, Eggleston JC, Spivak JL. Association between mediastinal germ cell tumors and hematologic malignancies. Report of two cases and review of the literature. Am J Surg Pathol 1985;9:23-30. [Crossref] [PubMed]
- Nichols CR, Roth BJ, Heerema N, et al. Hematologic neoplasia associated with primary mediastinal germ-cell tumors. N Engl J Med 1990;322:1425-9. [Crossref] [PubMed]
- Nichols CR. Mediastinal germ cell tumors. Semin Thorac Cardiovasc Surg 1992;4:45-50. [PubMed]
- deMent SH. Association between mediastinal germ cell tumors and hematologic malignancies: an update. Hum Pathol 1990;21:699-703. [Crossref] [PubMed]
- Chariot P, Monnet I, LeLong F, et al. Systemic mast cell disease associated with primary mediastinal germ cell tumor. Am J Med 1991;90:381-5. [Crossref] [PubMed]
- Chariot P, Monnet I, Gaulard P, et al. Systemic mastocytosis following mediastinal germ cell tumor: an association confirmed. Hum Pathol 1993;24:111-2. [Crossref] [PubMed]
- Orazi A, Neiman RS, Ulbright TM, et al. Hematopoietic precursor cells within the yolk sac tumor component are the source of secondary hematopoietic malignancies in patients with mediastinal germ cell tumors. Cancer 1993;71:3873-81. [Crossref] [PubMed]
- Zon R, Orazi A, Neiman RS, et al. Benign hematologic neoplasm associated with mediastinal mature teratoma in a patient with Klinefelter’s syndrome: a case report. Med Pediatr Oncol 1994;23:376-9. [Crossref] [PubMed]
- Hartmann JT, Nichols CR, Droz JP, et al. Hematologic disorders associated with primary mediastinal nonseminomatous germ cell tumors. J Natl Cancer Inst 2000;92:54-61. [Crossref] [PubMed]
- Oshrine BR, Olsen MN, Heneghan M, et al. Acquired isochromosome 12p, somatic TP53 and PTEN mutations, and a germline ATM variant in an adolescent male with concurrent acute megakaryoblastic leukemia and mediastinal germ cell tumor. Cancer Genet 2014;207:153-9. [Crossref] [PubMed]
Cite this article as: Kakkar A, Kaur K, Verma A. Pediatric mediastinal germ cell tumors. Mediastinum 2019;3:30.


