
Induction chemotherapy for locally advanced thymic epithelial tumors: consideration from the RYTHMIC prospective cohort
Thymic epithelial tumors (TETs) are rare neoplasms that are highly heterogeneous, ranging from indolent to highly aggressive (1). Surgery has been the mainstay of treatment and complete resection of a tumor is one of the most important prognostic factors (2). Masaoka’s staging system has been widely used for a long time (3). According to this system, patients with stage I or II disease have an excellent prognosis (4). In 2017, the TNM staging system for TETs was established for the first time (5). In this system, Masaoka stages I and II were merged into a single stage I. Furthermore, tumor that invaded only the pericardium was reclassified as stage II. In this new staging system, patients with either stage I or stage II disease had an excellent prognosis. Meanwhile, even in patients with stage III or stage IV disease, the prognosis was still better than that in patients with lung cancer (6). The unique tumor biology and somewhat indolent behavior of TETs might contribute to these findings. However, the better prognosis of patients with advanced TET can be attributed to the establishment of multimodal therapy for these tumors (7-9).
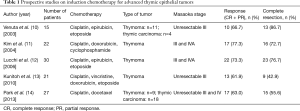
To date, there have been five prospective studies on induction chemotherapy for advanced TETs (Table 1) (10-14). Kunitoh and colleagues reported the results of weekly, dose-dense chemotherapy followed by surgery and/or thoracic radiotherapy for patients with unresectable locally advanced thymoma (13). They observed a response rate of 62% and a complete pathologic response rate of 14%. Surprisingly, 9 of 21 (43%) eligible patients underwent complete resection, although their tumors were initially judged to be unresectable by the multidisciplinary team. The other four prospective studies found similar results. However, since each study had different eligibility criteria, and perhaps differences in the degree of tumor spread, it is impossible to determine which treatment strategy is the most effective. Furthermore, there have been no randomized trials on the management of advanced TETs due to their rarity. Accordingly, treatment strategies for patients with advanced TET have been established based on such previous non-comparable studies. The latest NCCN guidelines recommend induction chemotherapy for patients with locally advanced TET that is potentially resectable (15). As mentioned below, the term “potentially resectable” is important in routine clinical practice.
Full table
A recent study by Merveilleux du Vignaux and colleagues assessed the efficacy of systemic treatments for patients with advanced TET who were included in a nationwide network (RYTHMIC) prospective database hosted by the French Thoracic Cancer Intergroup (16). They conducted analyses based on the actual intent of the treatment: primary chemotherapy (chemotherapy with the intent of subsequent surgical resection or definitive radiotherapy), exclusive systemic therapy (systemic therapy with no plan for surgery or radiotherapy), and systemic therapy for recurrence, for the first time. This classification was proposed by the International Thymic Malignancies Interest Group (ITMIG) (17). One of the most remarkable points of this article is the higher response rate for the primary chemotherapy group than in previous reports. The response rate to primary chemotherapy was 83% for patients with thymoma and 75% for those with thymic carcinoma. Meanwhile, in the five previous prospective studies, the response rate was 60% to 80% (Table 1). Furthermore, thymic carcinoma that tended to be refractory to chemotherapy was a minor entity in those studies. There are several possible explanations for the relatively high response rate in the study by Merveilleux du Vignaux. First, as the authors mentioned, they included patients who underwent multimodal therapy fairly recently; from 2012 to 2015. The tools available for radiologic evaluation before and after systemic therapy and the management of chemotherapy have both improved compared to those in previous studies. Second, the primary chemotherapy group may include more patients with resectable or potentially resectable disease than the exclusive systemic therapy group. If so, patients in the primary chemotherapy group could have a better physical status and thus be given more aggressive therapy, which could ultimately yield a better response rate. Thus, although other factors may have influenced the results of their study, the high response rates should strongly encourage thoracic oncologists, thoracic surgeons and other experts who treat patients with advanced TET.
As a more aggressive therapy, induction chemoradiotherapy followed by surgery has been an optional treatment strategy for locally advanced malignant tumors. For TETs, there has been only one prospective trial, by Korst and colleagues (18). In that study, induction therapy consisted of two cycles of cisplatin and etoposide with concurrent radiotherapy (45 Gy). Of the 21 patients, 17 (81%) underwent R0 resection, 3 (14%) underwent R1 resection, and 1 (5%) underwent debulking surgery. Five (24%) patients had a nearly complete response, and less than 10% of their tumors were viable. The authors concluded that induction chemoradiotherapy appeared to be feasible and showed a high rate of R0 resection. While this treatment strategy is only available for tumors that can be treated by radiotherapy, it is a promising option for locally advanced TETs. Currently, candidates for this treatment strategy should be carefully determined by a multidisciplinary team.
Recently, for many advanced malignancies, remarkable improvements in treatment efficacy have been achieved by targeted therapies in addition to conventional chemotherapy. Two studies examined the clinical responses to cetuximab in patients with advanced thymoma and suggested that this EGFR inhibitor is a promising therapeutic option (19,20). To explore the incorporation of cetuximab into a preoperative chemotherapy regimen, a phase II clinical trial of cetuximab with cisplatin, doxorubicin, and cyclophosphamide is currently underway in patients with locally advanced thymoma (NCT01025089). In terms of targeted therapies for thymic carcinoma, based on a phase II clinical trial, sunitinib has been recognized as a reasonable second-line treatment (21). At present, the Style trial (trial of sunitinib in patients with type B3 thymoma or thymic carcinoma in second and further lines, NCT03449173) is ongoing. In addition, second-line pembrolizumab was associated with a promising response rate and survival in patients with thymic carcinoma (22). The use of immune checkpoint inhibitors for patients with advanced TET is still under investigation (NIVOTHYM: NCT03134118). The role of these agents in induction therapy for advanced TTEs will also be investigated in the near future.
As exemplified by the study from Merveilleux du Vignaux and colleagues, studies that focus on the intent of chemotherapy can facilitate the comparison of several cohorts that include patients with advanced TET. Meanwhile, another practice that could promote the accurate comparison of several cohorts is the use of standardized definitions regarding the status of resectability: resectable, potentially resectable and unresectable. Of the five previous prospective studies mentioned above, 3 included patients with unresectable TET (Table 1). However, none of the five studies defined their inclusion criteria in detail. Resectability is generally judged based on the kinds and degrees of direct involvement of various mediastinal structures; i.e., “level 3” (lung, brachial vein, superior vena cava, chest wall, phrenic nerve) or “level 4” (aorta, myocardium, brachiocephalic artery, pulmonary artery) (23). Accordingly, it is difficult to clearly distinguish between resectable and potentially resectable as well as potentially resectable and unresectable. The eligibility criteria that were used in the study by Korst and colleagues (18) (tumor more than 8 cm in size, between 5 and 8 cm with multifocal calcification, heterogeneous appearance, irregular borders or great vessel invasion or encirclement, or less than 5 cm with great vessel invasion or encirclement) may be helpful for future trials on induction treatment for advanced TETs. Thus, clear criteria for the status of resectability that can be commonly and widely applied need to be established in the near future.
In conclusion, not only ongoing prospective clinical trials but also robust analyses of previous studies and studies using a large database of patients with advanced TET will lead to more effective therapeutic approaches for patients with advanced TET and improve their treatment outcomes.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by Section Editor Dr. Zhuoqi Jia (Thoracic Department, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China).
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med.2019.04.03). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Travis WD, Brambilla E, Burke AP, et al. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. 4th edition. Lyon: IARC, 2015.
- Zhu L, Zhang J, Marx A, et al. Clinicopathological analysis of 241 thymic epithelial tumors-experience in the Shanghai Chest Hospital from 1997-2004. J Thorac Dis 2016;8:718-26. [Crossref] [PubMed]
- Masaoka A, Monden Y, Nakahara K, et al. Follow-up study of thymomas with special reference to their clinical stages. Cancer 1981;48:2485-92. [Crossref] [PubMed]
- Nakagawa K, Asamura H, Matsuno Y, et al. Thymoma: A clinicopathologic study based on the new World Health Organization classification. J Thorac Cardiovasc Surg 2003;126:1134-40. [Crossref] [PubMed]
- Brierley JD, Gospodarowicz MK, Wittekind C. editors. TNM Classification of Malignant Tumours. 8th edition. New York: Wiley-Blackwell, 2017.
- Detterbeck FC, Stratton K, Giroux D, et al. The IASLC/ITMIG thymic epithelial tumors staging project: proposal for an evidence-based stage classification system for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol 2014;9:S65-72. [Crossref] [PubMed]
- Riely GJ, Huang J. Induction therapy for locally advanced thymoma. J Thorac Oncol 2010;5:S323-6. [Crossref] [PubMed]
- Hamaji M, Ali SO, Burt BM. A meta-analysis of induction therapy for advanced thymic epithelial tumors. Ann Thorac Surg 2015;99:1848-56. [Crossref] [PubMed]
- Berghmans T, Durieux V, Holbrechts S, et al. Systemic treatments for thymoma and thymic carcinoma: A systematic review. Lung Cancer 2018;126:25-31. [Crossref] [PubMed]
- Venuta F, Rendina EA, Longo F, et al. Long-term outcome after multimodality treatment for stage III thymic tumors. Ann Thorac Surg 2003;76:1866-72. [Crossref] [PubMed]
- Kim ES, Putnam JB, Komaki R, et al. Phase II study of a multidisciplinary approach with induction chemotherapy, followed by surgical resection, radiation therapy, and consolidation chemotherapy for unresectable malignant thymomas: final report. Lung Cancer 2004;44:369-79. [Crossref] [PubMed]
- Lucchi M, Melfi F, Dini P, et al. Neoadjuvant chemotherapy for stage III and IVA thymomas: a single-institution experience with a long follow-up. J Thorac Oncol 2006;1:308-13. [Crossref] [PubMed]
- Kunitoh H, Tamura T, Shibata T, et al. A phase II trial of dose-dense chemotherapy, followed by surgical resection and/or thoracic radiotherapy, in locally advanced thymoma: report of a Japan Clinical Oncology Group trial (JCOG 9606). Br J Cancer 2010;103:6-11. [Crossref] [PubMed]
- Park S, Ahn MJ, Ahn JS, et al. A prospective phase II trial of induction chemotherapy with docetaxel/cisplatin for Masaoka stage III/IV thymic epithelial tumors. J Thorac Oncol 2013;8:959-66. [Crossref] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology - thymomas and thymic carcinomas. Ver. 1. 2019. Available online: http://www.nccn.org/professionals/physician_gls/PDF/thymic.pdf. Accessed March 1, 2019.
- Merveilleux du Vignaux C, Dansin E, Mhanna L, et al. Systemic therapy in advanced thymic epithelial tumors: insights from the RYTHMIC prospective cohort. J Thorac Oncol 2018;13:1762-70. [Crossref] [PubMed]
- Girard N, Lal R, Wakelee H, et al. Chemotherapy definitions and policies for thymic malignancies. J Thorac Oncol 2011;6:S1749-55. [Crossref] [PubMed]
- Korst RJ, Bezjak A, Blackmon S, et al. Neoadjuvant chemoradiotherapy for locally advanced thymic tumors: a phase II, multi-institutional clinical trial. J Thorac Cardiovasc Surg 2014;147:36-44. [Crossref] [PubMed]
- Farina G, Garassino MC, Gambacorta M, et al. Response of thymoma to cetuximab. Lancet Oncol 2007;8:449-50. [Crossref] [PubMed]
- Palmieri G, Marino M, Salvatore M, et al. Cetuximab is an active treatment of metastatic and chemorefractory thymoma. Front Biosci 2007;12:757-61. [Crossref] [PubMed]
- Thomas A, Rajan A, Berman A, et al. Sunitinib in patients with chemotherapy-refractory thymoma and thymic carcinoma: an open-label phase 2 trial. Lancet Oncol 2015;16:177-86. [Crossref] [PubMed]
- Giaccone G, Kim C, Thompson J, et al. Pembrolizumab in patients with thymic carcinoma: a single-arm, single-centre, phase 2 study. Lancet Oncol 2018;19:347-55. [Crossref] [PubMed]
- Nicholson AG, Detterbeck FC, Marino M, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposals for the T Component for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol 2014;9:S73-80. [Crossref] [PubMed]
Cite this article as: Nakagawa K. Induction chemotherapy for locally advanced thymic epithelial tumors: consideration from the RYTHMIC prospective cohort. Mediastinum 2019;3:13.


