
Levels of Tc17 cells in thymic epithelial cell tumors associated with autoimmune diseases
Introduction
Thymomas are tumors derived from thymic epithelial cells, which generally grow slowly. Thymomas are the most common primary tumor in the anterior mediastinum (about 50%), and account for about 20% of mediastinal tumor incidence in adults (1). The thymic microenvironment plays a crucial role in the differentiation, proliferation and development of T lymphocytes. The development of thymic epithelial tumors is associated with a perturbed thymic microenvironment, while T cells are the major effector cells in the tumor microenvironment. Self-reactive T and B lymphocyte clones can be formed by the human immune system and attack autologous cells or their components, leading to autoimmunity. Persistent autoimmunity induces autoimmune disease (AD). Thymoma is associated with numerous ADs such as myasthenia gravis (MG), pure red cell aplasia (PRCA), hypogammaglobulinemia, systemic lupus erythematosus (SLE), Sicca syndrome, syndrome of inappropriate antidiuretic hormone secretion, pemphigus, and autoimmune thyroid disease (2). 40% of patients with thymomas have underlying ADs, with MG being the most common, making up about 20–25% of these patients. Moreover, 4–7% of patients with thymomas have two or more than two underlying ADs (3). Approximately 75% of MG patients have various degrees of thymus-related abnormalities, making up 85% of thymic hyperplasia cases and 15% of thymomas (4). Wang et al. (5) discovered through flow cytometry that the proportions of Th17 cells in the peripheral blood of MG patients with thymomas were higher than in healthy controls. There has been increasing interest in IL-17 and Th17 cells in recent years. A group of CD8+ T lymphocytes in the body are characterized by high-level IL-17 secretion (6). These cells were defined as a new subtype of cytotoxic T cell (CTL) in 2009 and called Tc17 cells (7-9) as an abbreviated form of IL-17-secreting CD8+ effector cells. The differentiation, phenotype and function of Tc17 cells are distinctly different from those of other canonical CTLs but share similarities with Th17 cells. There is now some appreciation that research on IL-17 should not only focus on CD4+ T cells, but also on IL-17-secreting CD8+ Tc17 cells. Several studies have addressed the involvement of Tc17 cells in ADs and tumor development and showed that Tc17 cell levels in tumors or patients with ADs were remarkably increased. However, the role of Tc17 cells in thymic epithelial tumors, especially in patients with MG and other ADs, has not been examined.
Studies of Th17/Tc17 cell involvement in several diseases are ongoing, and more attention is now being paid to the influence of the Th17/Tc17 ratio on disease. However, no intensive study of thymic epithelial tumors in patients with MG and other ADs is currently available. Retinoid-related orphan receptor-γt (RORγt) is the characteristic transcription regulatory factor of Tc17 cells (10). Upregulation and functional activation of RORγt promotes the differentiation of CD8+ T lymphocytes into Tc17 cells (6,11).
In this study, we assessed expression of the critical regulatory factor RORγt in thymic epithelial tumor tissue using RT-PCR and analyzed the association of Tc17/Th17 cells with ADs. Levels of Tc17/Th17 cells in thymic epithelial tumors were assessed by flow cytometry to explore the roles of these cells in thymic epithelial tumors of patients with ADs and their clinical significance.
Methods
Study subjects
Case source
Patients undergoing thymic epithelial tumor resection at Tianjin Medical University General Hospital from March 2010 to June 2016 were selected.
Diagnostic criteria
Diagnosis was confirmed based on clinical symptoms (skeletal muscle involvement, pathological muscle fatigue mild at dawn and severe at sunset, and remission after rest) as well as neostigmine test (intramuscular injection of 1.5 mg neostigmine could markedly alleviate the above symptoms, with the greatest effect being achieved at 30 min and lasting for 1–2 h). The ADs in MG patients according to PubMed Health included type I diabetes, immune-related thyroid dysfunction, multiple dermatomyositis, SLE, PRCA and Sicca syndrome. The antinuclear antibody ratios of MG patients were >1:80.
Case data
Enrolled patients were divided into three groups: (I) patients with thymic epithelial tumors only (Tm group); (II) patients with thymic epithelial tumors combined and MG (MG group); and (III) patients with thymic epithelial tumors as well as MG and other ADs or abnormally elevated antinuclear antibody ratios (AD group). In total, 54 patients with thymic epithelial tumors were enrolled with an average age of 52.76±10.8 years (range, 26 to 73 years). Twenty-seven patients were male and 27 were female. According to the Masaoka classification, 19 cases were stage I, 21 cases were stage II, 12 cases were stage III and 2 cases were stage IV. Based on the Osserman clinical classification, 14 cases were type I, 24 cases were type II (9 cases of IIa and 15 cases of IIb), 7 cases were type III and none were type IV. Thymic epithelial tumor tissue specimens were grouped according to WHO pathological classification and included 3 specimens of type A, 7 specimens of type AB, 40 specimens of type B (14 B1, 17 B2 and 9 B3), and four specimens of type C (Table 1).
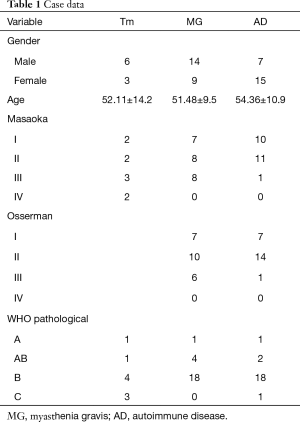
Full table
Experimental procedures and methods
Detection of clinical immune markers: CD3+ T cells, CD4+ T cells, CD8+ T cells, interferon (IFN)-γ and interleukin (IL)-4
Flow cytometry of CD3+ T cells, CD4+ T cells, CD8+ T cells, interferon (IFN)-γ and interleukin (IL)-4: Fc receptors were blocked after harvesting cells followed by cell surface staining, fixation, rupture of the cell membrane and intracellular staining. Finally, cells were analyzed using the flow cytometer.
Detection of clinical immune markers: levels of IgG, IgA, IgM, C3, C4, C-reactive protein (CRP), immune complexes and IgE were measured by ELISA
From each envelope antigen: 0.3 mL has diluted with liquid package is to the best concentration of reaction plate is a kind of antigen to each of the concave hole, 4 °C for the night; wash 3 times; add checked samples: each join in concave hole contains 0.05% twain—20 has been screening serum dilution of 0.2 mL, 37 °C, 1–2 hours incubation;After 3 times of washing, enzyme binding was added: 0.2 mL of diluted enzyme binding was added into each cavity. 1–2 hours 37 °C effect: after three times to 0.2 mL substrate solution in each of the concave hole (O.P.D or O.T), incubation for 30 minutes at room temperature (ck: 0.4 mL substrates to join 0.1 mL termination agent); add termination agent: add 2M citric acid 0.05 mL for each pit.Finally, the results were observed and recorded. The O.D value was determined by enzyme—labeled colorimeter.
Detection of Tc17 cells in thymic tissue by double staining
Double staining can be used to show two antibodies in different locations, tissues or cells. For instance, one may be expressed on the cell membrane, while another may be expressed in intercellular space. Staining by two antibodies was detected in this experiment using streptavidin-biotin labeling. The two distinct color development systems employed in this experiment were the nitroblue tetrazolium-alkaline phosphatase system and the 3-amino-9-ethylcarbazole-peroxidase system. The formed yielded a black-blue color, while the latter yielded red color. Mouse anti-human CD8 monoclonal antibody (Abcam, USA) and rabbit anti-human IL-17A monoclonal antibody (Abcam, USA) were used in the experiment. The reference negative control was set, and positive Tc17 cells showed black blue and red morphology based on hematoxylin and eosin staining sections. Thymic epithelial tumor immunohistochemical staining sections were observed under a light microscope, and the number of positive cell in each high power field was calculated macroscopically and under light microscopy.
Quantitation of RORγt mRNA levels in thymus by RT-PCR
Total RNA in thymic tissue was extracted and reverse transcribed into cDNA for PCR amplification. Premier Primer 5 software was used for automatic search and Oligo 6 software was used for analysis and evaluation. Primers were designed using conventional principles (Table 2). PCR reactions were conducted in a final volume of 25 µL. PCR products were analyzed using gel electrophoresis with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as the internal reference. Double-distilled water was used as the template for negative control reactions. The same primers were used for real-time quantitative PCR.
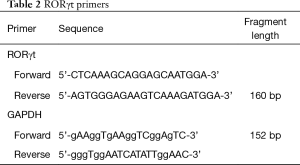
Full table
Peripheral blood Th17/Tc17 cell quantitation by flow cytometry
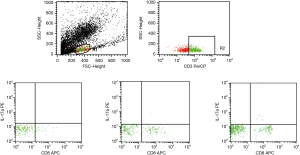
Blood samples were obtained and labeling was conducted after stimulation and activation using CD3-PerCP (IQ Products, USA) and CD8-APC (IQ Products, USA) antibodies. Subsequently, IL-17A-PE (BD, USA) antibody was added for labeling after hemolysis, rupture of membranes and permeabilization. Subsequently, 0.5 mL of phosphate-buffered saline was added and mixed, followed by loading and detection. CD3+CD8-IL17+ cells were defined as Th17 cells, while CD3+CD8+IL17+ cells were defined as Tc17 cells.
Statistical analysis
The relative expression of target mRNA was calculated as the ratio of target band gray-scale value/GAPDH gray-scale value. Positive cell ratios were expressed as the number of positive cells in the field of view divided by the total number of cells in the field of view. Both of these figures were expressed as means ± standard deviations (). Multiple groups of data were compared using the chi-square test, and means of two groups were compared using the independent sample t test. SPSS 19.0 software was used for all statistical analyses. Statistical significance was assumed for P<0.05.
Results
Clinical immune indexes in patients with thymic epithelial tumors
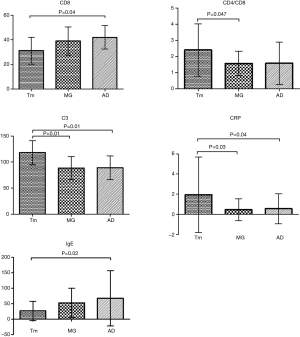
We collected clinical data including sex, age, and modified Ossermann classification of MG patients. The laboratory data were mainly counts of peripheral CD3+, CD4+ and CD8+ T cells and serum levels of IFN-γ, IL-4, IgG, IgA, IgM, C3, C4, CRP and anti-nuclear antibody (Table 3, Figure 1).
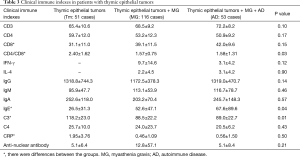
Full table
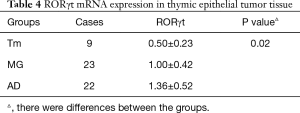
RORγt mRNA expression levels in thymic epithelial tumors
Semi-quantitative results
Relative gray values were calculated. Difference in RORγt mRNA levels in thymic epithelial tumor tissue between patients with thymic epithelial tumors alone (0.50±0.23) and thymic epithelial tumors as well as MG (1.00±0.42) were statistically significant (P<0.05). Differences in RORγt mRNA expression in thymic epithelial tumor tissue between patients with thymic epithelial tumors alone (0.50±0.23) and thymic epithelial tumors as well as MG and elevated anti-nuclear antibodies or ADs (1.36±0.52) (P<0.05) were statistically significant (P<0.05). Among MG patients with thymic epithelial tumors, RORγt mRNA expression was similar in those with elevated anti-nuclear antibody or ADs (1.36±0.52), showing no statistically significant difference (P>0.05) (Figure 2, Table 4).
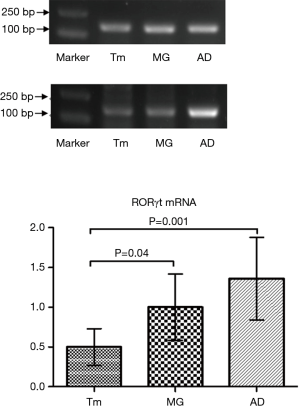
Full table
Quantitative PCR results
The results were further verified through quantitative PCR, which confirmed that RORγt mRNA expression in the MG and AD groups was upregulated compared with the Tm group (Figure 3).
Association between RORγt mRNA expression and clinical features
The relative gray-scale values were determined. Difference in RORγt mRNA expression in thymic tissue between male and female patients were not statistically significant. However, RORγt mRNA expression in patients with mild and severe Osserman clinical classifications differed significantly (P<0.05). Moreover, RORγt mRNA levels gradually increased in patients with severe clinical classification. Difference in RORγt mRNA levels differed significantly in patients with different WHO classifications (P<0.05). RORγt mRNA expression levels were notably higher in patients with thymic epithelial tumors of types C, B2 and B3 compared with those of types A, AB and B1. No significant differences in RORγt mRNA expression were observed among thymic epithelial tumors at different Masaoka stages (P>0.05) (Table 5).
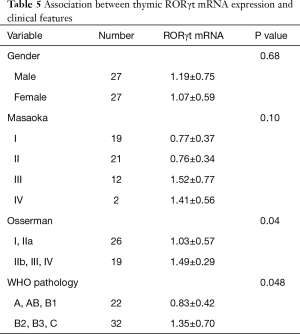
Full table
IL-17 was expressed in the cytoplasm or was secreted into the intercellular space. Positive Tc17 cells showed black-blue and red morphologies and were located in lymphocyte areas. Positive cells were counted under a microscope, and we observed that Tc17 cells were mainly lymphocytes. Histological classification of thymic epithelial tumors is related to the proportion of epithelial cells among lymphocytes; therefore, thymic epithelial tumors of various classifications had some influence on the quantity of Tc17 cells. The proportion of Tc17 cells in the Tm group was significantly lower than in the MG group and the AD group (P<0.05). The distribution of positive Tc17 cells in the MG group was similar to that of the AD group, but no differences were observed between the MG and AD groups (P>0.05) (Figure 4, Table 6).
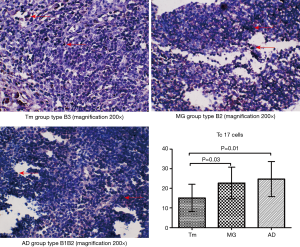
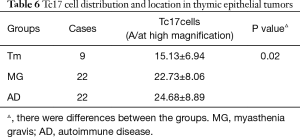
Full table
Association between thymic Tc17 cell distribution and clinical features
Positive Tc17 cells were counted and compared with the clinical features of patients. This comparison is shown in the table below (Table 7).
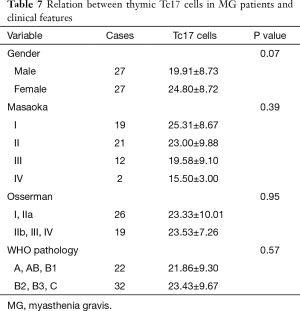
Full table
Levels of peripheral blood Th17/Tc17 cells in patients with thymic epithelial tumors
Tc17 cells were defined as CD3+CD4-CD8+IL17+ T cells while Th17 cells were defined as CD3+CD4+CD8-IL17+ T cells. Compared with Tm group, overall T-cell levels were higher in both the MG group and the AD group. Moreover, among T-cells, levels of Th17 and Tc17 cells also showed an increasing trend in the MG and AD group compared with the Tm group (Figure 5).
Discussion
William Coley discovered the interaction between tumor cells and the immune system while treating advanced sarcoma in the 1890s. The role of tumors in autoimmunity at the active stage remains unclear. In particular, whether thymomas can trigger mature long-lasting T cells to assist in antibody production in the peripheral blood remains unclear.
Association between immune indexes in patients with thymic epithelial tumors and AD
Research on peripheral blood T lymphocyte subsets, immunoglobulin and complement changes has primarily focused on ADs such as rheumatoid arthritis (RA), IgA nephropathy, liver disease and leucoderma. The CD4+/CD8+ T cell ratios of RA patients are elevated (12). IgG, IgM and IgA levels are also elevated with disease activity, especially IgG (13). The ratios of various T lymphocyte subsets in blood are completely abnormal in RA patients (14). Moreover, plasma complement C3 levels gradually decrease as the disease progresses from mild to severe, and also gradually decease with disease activity (9). Serum C3 levels in leucoderma patient are lower than in healthy subjects. However, differences in IgG, IgA, IgM and C4 levels between leucoderma patients and healthy subjects were not statistically significant (15). In our study, IgE in the AD group was significantly elevated. IgE has the lowest levels of all immunoglobulins in the human body, but is increased during type I hypersensitivity reactions and has high affinity for mast cells and basophils.
Elevated CD4+/CD8+ ratios can mainly be observed in diseases such as adenocarcinoma of the lung, squamous cell carcinoma, active SLE, and RA. Thus, CD4+/CD8+ ratios are closely related to clinical disease, especially in ADs and cancer. In our study, CD8+ T-cell levels were significantly elevated in the AD group, and the CD4+/CD8+ ratio decreased. Therefore, CD8+T cell plays a crucial role in ADs. This indicates that CD8+ Tc17 cells play an important role in ADs. The CD4+/CD8+ ratio can be used for early clinical screening.
In our study, IgE levels in the MG and AD groups were markedly higher than that in the Tm group, while the C3 levels were lower than in the Tm group. Scadding et al. (16) analyzed immune indexes in MG patients after thymoma surgery. They found that AchR antibody was distinctly lower compared with before surgery, and that IgM and IgE levels were reduced post-surgery. Liu et al. (17) pointed out that C3 levels were negatively correlated with disease severity in patients with systemic MG with positive AChR autoantibody. Therefore, monitoring the C3 level in these patients allows for timely treatment, which can avoid myasthenia crisis. However, C3 level is not correlated with disease severity in systemic MG patients with negative AChR autoantibody. Based on these results, MG patients appear to have decreased serum C3 levels, which may be attributed to immune complex formation in these patients. This results in complement activation, thus increasing complement consumption and playing an important role in the pathogenesis of MG. Detecting changes in total complement activity or levels of specific complement factors in serum is of great clinical significance in the diagnosis, progression and treatment of related diseases.
Relation between Th17/Tc17 cells and thymic epithelial tumors in patients with ADs
AD status
The presence of thymic epithelial tumors alters the thymic microenvironment and can disorder T cell development. This may result in development of immature T-cells and immune tolerance deficits. Immature lymphocytes cannot identify autoantigens, instead taking them for alloantigens, attacking them and leading to AD.
A large number of cortical thymic lymphocytes in thymomas proliferate rapidly, which may significantly add to the possibility of gene mutation (18). Theofilopoulos et al. (19) discovered that thymic cortex-derived cells had self-reactivity during rapid proliferation. In addition, Okumura et al. (20) suggested that AD in thymoma patients was related to the downregulation of HLA-DR expression in thymic epithelial cells within the thymoma. ADs such as MG mainly occur in patients with type B1, B2 and AB thymomas in which a large number of CD4+CD8+ double positive (DP) cells are present. These cells cannot identify antigens and have no function, apparently representing T cells that have not completed development. Downregulated HLA-DR expression in thymic epithelial cells within the thymoma results in positive selection disorder in CD4+CD8+ DP cells. Furthermore, production of CD4+CD8− single positive cells with immune function is reduced, which eventually leads to self-reactivity.
The role of Tc17 cells in AD
Interest is increasing in IL-17 and Th17 cells. A group of CD8+T lymphocytes in the body are characterized by IL-17 secretion (6) and were defined as a new CTL subtype in 2009 called Tc17 cells (7-9). The differentiation, phenotype and function of Tc17 cells differ from those of other canonical CTLs. Tc17 cells have properties that are similar to those of CD4+ cells, which cooperate with humoral immunity through secreting interleukins. As a result, these cells are speculated to be a bridge connecting tumors with immunity, and may play a vital role in development of tumors as well as ADs.
IL-17 is key to Th17/Tc17 cell function. IL-17 can bridge the adaptive immune response with innate immune responses by mediating neutrophil mobilization, thus playing a key role in defense against microbial infection. Meanwhile, abnormal IL-17 expression is closely related to chronic inflammation and AD. IL-17 is a characteristic cytokine of Tc17 and Th17 cells. It serves as a pro-inflammatory factor and can upregulate chemokines and metalloproteins to recruit neutrophils to sites of inflammation (21). However, the function of an entire cell cannot be determined from the function of a single inflammatory factor. IL-17+ T lymphocytes can also secrete cytokines like IL-21 and IL-23, which also play important roles in cellular immunity.
The role of tumors in autoimmunity at the active stage remains unclear. In particular, whether thymomas can induce mature long-lived T cells to assist in antibody production in peripheral blood remains unclear. Buckley et al. (22) discovered from their studies of T-cell receptor excision circles (TRECs) that, compared with healthy controls or delayed myasthenia patients, MG patients with thymomas had elevated TREC levels in peripheral CD4+ and CD8+ cells. However, TRECs in thymoma patients were only elevated in CD8+ cells. TREC levels were reduced after thymoma resection, but increased again at thymoma recurrence. This result supported the hypothesis that thymomas can induce mature and long-existing T cells; moreover, these T cells reflect the pathological process of thymoma formation and may be associated with AD.
RORγt is a critical transcription factor of Tc17 cells, which can induce expression of IL-17 and IL-17F genes, thus regulating IL-17 (9). In the presence of a small amount of transforming growth factor-β, IL-6 or IL-21 can induce production of a large amount of RORγt in a signal transducer and activator of transcription 3 (STAT3)-dependent manner. STAT3 is activated by IL-6, IL-21 and IL-23, and plays an important regulatory role in IL-17 production by T cells. On the one hand, STAT3 can upregulate ROR-γt expression, thus affecting IL-17 expression. On the other hand, STAT3 can also directly bind to the IL-17 and IL-21 promoters. In vitro induction of the differentiation of CD8+ T cells into Tc17 cells depends on the transcription factors RORγt and FOXP3 rather than Eomes or T-bet (7). In contrast, Tc1 and Tc2 differentiation depend on Eomes and T-bet, rather than RORγt or FOXP3. Eomes and T-bet can regulate the differentiation of natural killer and CD8+ T cells (23). FOXP3 plays a major regulatory role in producing T cells and Treg cells. In vivo and in vitro studies have indicated that upregulation of Tc17 cell differentiation and suppression of CTL differentiation are dependent on STAT3. This suggests the mutually exclusive nature of Tc17 cell and canonical CTL cell differentiation (9).
Garcia-Hernandez et al. (24) transferred Tc17 cells induced in vitro in a murine model of melanoma. They discovered that Tc17 cells could recruit immune cells such as neutrophils and macrophages to tumor sites by producing IL-17, IFN-γ and tumor necrosis factor (TNF). As a result, Tc17 cells could induce tumor cell apoptosis, inhibit tumor growth, and exert important antitumor effects. In AD research, Huber et al. (25) pointed out that Tc17 cells could support Th17 cell-mediated canonical AD in a model of experimental autoimmune encephalomyelitis. Moreover, Tc17 cells played a vital role in the initial stages of autoimmune nervous system disease. In addition, it was pointed out that IFN regulatory factor could control Tc17 cell differentiation through balancing RORγt, Eomes and Foxp3. The proportion of Tc17 cells in cerebrospinal fluid is much higher than that in peripheral blood, and thus Tc17 cells may act primarily at sites of disease. Consequently, Tc17 cells play a crucial role in cancer and autoimmunity. Therefore, a critical role of Tc17 cells in thymoma formation in patients with ADs is undisputed.
In a model of experimental autoimmune MG, Th17 cell proportions were markedly increased as disease progresses, accompanied with an increase in IL-17 level (26). However, clinical research has suggested that Th17 cell proportions in MG patients are similar to those of healthy controls (27). Wang et al. (5) showed a result that conflicted with the clinical data. They found that Th17 cells were clearly elevated in MG patients with thymomas, but that differences among healthy controls, patients with thymic hyperplasia and MG patients with a normal thymus were not statistically significant. A recent study indicated that serum IL-17 levels were greatly increased in patients with systemic MG compared with patients with ocular MG and healthy controls. Furthermore, IL-17 level is correlated with AChR antibody (28). Thus, it can be concluded that IL-17 plays a role in thymoma formation in MG patients.
Lopes et al. (29) discovered through flow cytometry and RT-PCR that Th17 cell levels in spontaneous urticaria patients were reduced compared with healthy controls, while Tc17 cell levels were elevated. Therefore, they suggested that CTLs were the major participant in such autoimmune reactions. T cells would produce an inflammatory factor disorder, which led to systemic inflammation. However, in this experiment, RORγt expression in patients with thymomas combined with ADs was remarkably higher than in patients with thymomas alone. RORγt can regulate Th17 and Tc17 cells, demonstrating that Tc17 cells may be the major factor influencing RORγt expression in patients with thymomas and underlying ADs.
Th17/Tc17 cells play unique roles in local inflammation. Cells producing IL-17 during such processes have similar biological heterogeneity, and produce pro-inflammatory cytokines including IL-17, TNF-α and IL-2. Moreover, some cells will secrete IFN-γ, potentially after developing properties similar to Th1/Tc1 and Th2/Tc2 cells under certain environment conditions. Henriques et al. (30) had also investigated this idea in RA patients. TNF-α can kill target cells, promote apoptosis, and participate in local inflammation and endothelial cell activation. IL-2 can stimulate NK cells and macrophages and promote CTL function. IFN-γ can enhance MHC expression and antigen presentation, promote Th1 cell differentiation, suppress Th2 cell differentiation and promote CTL function. Therefore, we speculate that such a mechanism exists in Th17/Tc17 cells in patients with thymomas combined with ADs, such that Th17/Tc17 cells not only depend on the effects of IL-17. Moreover, the involvement of TNF-α, IL-2 and IFN-γ has complicated the mechanism of action of Th17/Tc17 cells. CD4+ T and CD8+ T cell differentiation is restricted by MHC molecules. IFN-γ can regulate MHC molecules, thus indirectly regulating the differentiation of Th17 and Tc17 cells. IL-2 and TNF-α can promote inflammation, further upregulating CTL differentiation. CTLs can bind antigen on tumor cells presented by MHC class II molecules, thus identifying and killing tumor cells. Thus, Tc17 cells manifest both CTL and Th cell functions and are involved in the genesis and development of ADs. Consequently, CTLs (and Tc17 cells) play a more prominent role, which is consistent with our results.
Significance of the Th17/Tc17 cell proportion
Our PCR experiments suggest a significant role of RORγt in patients with thymomas and ADs. Changes in the Th17/Tc17 cell proportion in the peripheral blood are under further study, and indicate statistically significant differences in this proportion among the Tm, MG and AD groups. Yaochite et al. (31) studies the pathogenesis of autoimmune diabetes and found that a balanced Th17/Tc17 cell proportion is the key to protect immune tolerance and prevent AD. Hoffacker et al. (32) demonstrated that thymus-derived auto-reactive T lymphocyte could activate CD4+ T cells to complete the transition from cellular immunity to humoral immunity, thus further activating B lymphocytes to produce corresponding antibody. A large amount of autoimmune T lymphocytes are produced in thymomas, which mark the initiation of AD when they enter the peripheral blood. Among thymoma patients with AD, those with MG and SLE have reduced autoimmune antibody titers after thymoma resection (33-36), which has further supported this idea.
However, a number of studies have found that thymoma patients with MG are mostly AChR autoantibody negative, indicating that AChR autoantibody is not the only factor in thymoma formation. Furthermore, the MG involvement site is not restricted to neuromuscular junctions; instead, the entire signal transduction pathway from axon to skeletal muscle and cell may be involved (37). Our results suggest that Tc17 cell levels in thymoma tissue in patients with MG and other ADs are notably higher than in patients with thymomas alone. This indicates that Tc17 cells may play a crucial role in immune disease. In addition, Tc17 cell levels in the AD group were higher than that in the MG group, but this difference was not statistically significant. Nevertheless, our data support the function of Tc17 cells in AD, which may be related to disease course. Tc17 cells in peripheral blood showed a similar trend, demonstrating that Tc17 cells not only play a role in the immune response in tissue, but can also regulate immunity in the peripheral blood. Tc17 cells can regulate expression of chemokines and chemokine receptors such as CCR7, and thus Tc17 cells in thymoma tissue can exert antitumor effects. However, Tc17 cells can also express CCR6 to recruit inflammatory cells, thereby enhancing inflammation and autoimmunity. Using the Osserman classification, RORγt mRNA levels in patients with mild and severe MG were significantly different. This result further revealed the significance of Th17/Tc17 cells in MG progression, and their presence may be a marker of disease progression. Alternatively, Tc17 cells may represent a crucial factor in patients with negative auto-Musk antibody and negative AchR autoantibody.
The number of peripheral blood samples analyzed by flow cytometry in this study was insufficient for statistical analysis. Nonetheless, we could deduce from our results that the levels of Tc17 and Th17 cells in patients with thymomas alone were markedly lower than those in patients with thymomas and MG or thymomas and MG and other ADs. This suggests that Tc17 and Th17 cells are involved in disease genesis and development in thymoma patients with MG and other ADs. This phenomenon further verifies our results in tissue, demonstrating the roles of Tc17 and Th17 cells in the systemic response. The thymus is the starting point of the systemic response, which is also the center of Tc17 and Th17 cells in peripheral blood. This work has provided a novel therapeutic thinking and theoretical foundation for thymomas in patients with MG and ADs. Currently, thymectomy is the primary means for treating MG. Further intensive investigation of the relationship between central and peripheral expression of Tc17 and Th17 cells and their roles in disease genesis and development will provide new therapeutic avenues for AD.
In summary, levels of Th17/Tc17 cells differed in patients with thymomas and MG and ADs compared with patients with thymomas alone. This difference was observable in peripheral blood, tissue and via RORγt mRNA levels. Our results indicated that Th17/Tc17 cells are involved in tumor immunity toward thymomas as well as the genesis and development of ADs. These cells play critical roles as mediators of cancer and autoimmunity. The role of Tc17 cells during such processes is crucial. Tc17 cells are a member of the CTL family which have similar properties to Th cells. Improved understanding of the role of Th17/Tc17 cells in thymomas in patients with ADs may help uncover novel diagnostic and therapeutic pathways.
Conclusions
CD8+ T cells, CD4+/CD8+ T cell ratio, and levels of complement C3, CRP and IgE are closely associated with thymoma in the setting of abnormal autoimmunity. The precise mechanisms underlying this process require further study. Tc17 cells may be used as a clinical early screening indicator to reveal the occurrence and development of ADs. Abnormal levels of Tc17 cells are closely linked with the pathogenesis of thymic epithelial tumors accompanying MG and other ADs. Abnormal expression of RORγt in thymic epithelial tumor tissue may be the major cause of autoimmune dysregulation, and its upstream and downstream regulatory pathways need to be further studied. Th17/Tc17 cells play a vital role in central immune organs, but are also abnormally distributed in peripheral blood. Our study has further demonstrated that Th17/Tc17 cells are important participants in the genesis and development of thymic epithelial tumors in patients with ADs. In the future, Tc17 cells can be used as a therapeutic target for patients with thymic epithelial tumors and ADs.
Acknowledgments
Funding: Major Science and Technology Project and Major Science and Technology Project of Chronic Disease Prevention of Tianjin (16ZXMJSY00030), Comprehensive diagnosis and treatment strategy of thymus-related autoimmune diseases. National Natural Science Foundation of China Youth Program (81801603), Pathogenesis of thymoma associated myasthenia gravis: TLR4 signaling activity induces imbalance of Th17 and Treg.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med.2019.02.02). PZ serves as an unpaid editorial board member of Mediastinum from May 2017 to Apr 2019. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained individual patients, and the experimental protocol was approved by the Clinical Research Ethics Committee of the Tianjin Medical University.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Annessi V, Paci M, De Franco S, et al. Diagnosis of anterior mediastinal masses with ultrasonically guided core needle biopsy. Chir Ital 2003;55:379-84. [PubMed]
- Evoli A, Minicuci GM, Vitaliani R, et al. Paraneoplastic diseases associated with thymoma. J Neurol 2007;254:756-62. [Crossref] [PubMed]
- Shelly S, Agmon-Levin N, Altman A, et al. Thymoma and autoimmunity. Cell Mol Immunol 2011;8:199-202. [Crossref] [PubMed]
- Grob D, Brunner N, Namba T, et al. Lifetime course of myasthenia gravis. Muscle Nerve 2008;37:141-9. [Crossref] [PubMed]
- Wang Z, Wang W, Chen Y, et al. T Helper Type 17 Cells Expand in Patients with Myasthenia-Associated Thymoma. Scand J Immunol 2012;76:54-61. [Crossref] [PubMed]
- Liu SJ, Tsai JP, Shen CR, et al. Induction of a distinct CD8 Tnc17 subset by transforming growth factor-β and interleukin-6. J Leukoc Biol 2007;82:354-60. [Crossref] [PubMed]
- Hamada H, Garcia-Hernandez Mde L, Reome JB, et al. Tc17, a Unique Subset of CD8 T Cells That Can Protect against Lethal Influenza Challenge. J Immunol 2009;182:3469-81. [Crossref] [PubMed]
- Kondo T, Takata H, Matsuki F, et al. Cutting edge: Phenotypic characterization and differentiation of human CD8+ T cells producing IL-17. J Immunol 2009;182:1794-8. [Crossref] [PubMed]
- Huber M, Heink S, Grothe H, et al. A Th17-like developmental process leads to CD8. Eur J Immunol 2009;39:1716-25. [Crossref] [PubMed]
- Zhao Y, Balato A, Fishelevich R, et al. Th17/Tc17 infiltration and associated cytokine gene expression in elicitation phase of allergic contact dermatitis. Br J Dermatol 2009;161:1301-6. [Crossref] [PubMed]
- Zhou L, Ivanov II, Spolski R, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol 2007;8:967-74. [Crossref] [PubMed]
- Yi H, Zhen Y, Jiang L, et a1. The phenotypic characterization of naturally occurring regulatory CD4+CD25+T cells. Cell Mol lmmunol 2006;3:189-95.
- Hsieh CS, Zheng Y, Liang Y. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat Immunol 2006;7:401-10. [Crossref] [PubMed]
- Zheng Y, Josefowicz SZ, Kas A. Nature 2007;445:936-40. [Crossref] [PubMed]
- Askennsy N, Kaminitz A, Yarkoni S. Mechanisms of T regulatory cell function. Autoimmun Rev 2008;7:370-5. [Crossref] [PubMed]
- Scadding GK, Webster AD, Ross M, et al. Humoral immunity before and after thymectomy in myasthenia gravis. Neurology 1979;29:502-6. [Crossref] [PubMed]
- Liu A, Lin H, Liu Y, et al. Correlation of C3 level with severity of generalized myasthenia gravis. Muscle Nerve 2009;40:801-8. [Crossref] [PubMed]
- Shoenfeld Y, Lorber M, Yucel T, et al. Primary antiphospholipid syndrome emerging following thymectomy for myasthenia gravis: additional evidence for the kaleidoscope of autoimmunity. Lupus 1997;6:474-6. [Crossref] [PubMed]
- Theofilopoulos AN, Dummer W, Kono DH. T cell homeostasis and systemic autoimmunity. J Clin Invest 2001;108:335-40. [Crossref] [PubMed]
- Okumura M, Fujii Y, Shiono H, et al. Immunological function of thymoma and pathogenesis of paraneoplastic myasthenia gravis. Gen Thorac Cardiovasc Surg 2008;56:143-50. [Crossref] [PubMed]
- Weaver CT, Hatton RD, Mangan PR, et al. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol 2007;25:821-52. [Crossref] [PubMed]
- Buckley C, Douek D, Newsom-Davis J, et al. Mature, long-lived CD4+ and CD8+ T cells are generated by the thymoma in myasthenia gravis. Ann Neurol 2001;50:64-72. [Crossref] [PubMed]
- Intlekofer AM, Takemoto N, Wherry EJ, et al. Effector and memory CD8+T cell fate coupled by T-bet and eomesodermin. Nat Immunol 2005;6:1236-44. [Crossref] [PubMed]
- Garcia-Hernandez ML, Hamada H, Reome JB, et al. Adoptive Transfer of Tumor-Specific Tc17 Effector T Cells Controls the Growth of B16 Melanoma in Mice. J Immunol 2010;184:4215-27. [Crossref] [PubMed]
- Huber M, Heink S, Pagenstecher A, et al. IL-17A secretion by CD8+ T cells supports Th17-mediated autoimmune encephalomyelitis. J Clin Invest 2013;123:247-60. [Crossref] [PubMed]
- Mu L, Sun B, Kong Q, et al. Disequilibrium of T helper type 1, 2and 17 cells and regulatory T cells during the development of experimental autoimmune myasthenia gravis. Immunology 2009;128:e826-36. [Crossref] [PubMed]
- Masuda M, Matsumoto M, Tanaka S, et al. Clinical implication of peripheral CD4 + CD25 + regulatory T cells and Th17 cells in myasthenia gravis patients. J Neuroimmunol 2010;225:123-31. [Crossref] [PubMed]
- Roche JC, Capablo JL, Larrad L, et al. Increased serum interleukin-17 levels in patients with myasthenia gravis. Muscle Nerve 2011;44:278-80. [Crossref] [PubMed]
- Lopes A, Machado D, Pedreiro S, et al. Different frequencies of Tc17/Tc1 and Th17/Th1 cells in chronic spontaneous urticaria. Int Arch Allergy Immunol 2013;161:155-62. [Crossref] [PubMed]
- Henriques A, Gomes V, Duarte C, et al. Distribution and functional plasticity of peripheral blood Th(c)17 and Th(c)1 in rheumatoid arthritis. Rheumatol Int 2013;33:2093-9. [Crossref] [PubMed]
- Yaochite JN, Caliari-Oliveira C, Davanso MR, et al. Dynamic changes of the Th17/Tc17 and regulatory T cell populations interfere in the experimental autoimmune diabetes pathogenesis. Immunobiology 2013;218:338-52. [Crossref] [PubMed]
- Hoffacker V, Schultz A, Tiesinga JJ, et al. Thymomas alter the T-cell subset composition in the blood: a potential mechanism for thymoma-associated autoimmune disease. Blood 2000;96:3872-9. [PubMed]
- Mygland A, Vincent A, Newsom-Davis J, et al. Autoantibodies in thymoma-associated myasthenia gravis with myositis or neuromyotonia. Arch Neurol 2000;57:527-31. [Crossref] [PubMed]
- Suzuki S, Utsugisawa K, Nagane Y, et al. Three types of striational antibodies in myasthenia gravis. Autoimmune Dis 2011;2011:740583 [Crossref] [PubMed]
- Zisimopoulou P, Brenner T, Trakas N, et al. Serological diagnostics in myasthenia gravis based on novel assays and recently identified antigens. Autoimmun Rev 2013;12:924-30. [Crossref] [PubMed]
- Soltys J, Gong B, Kaminski HJ, et al. Extraocular muscle susceptibility to myasthenia gravis: unique immunological environment? Ann N Y Acad Sci 2008;1132:220-4. [Crossref] [PubMed]
- Liang Y, Pan HF, Ye DQ. IL-17A-producing CD8(+)T cells as therapeutic targets in autoimmunity. Expert Opin Ther Targets 2015;19:651-61. [Crossref] [PubMed]
Cite this article as: Li J, Chen Y, Wang Y, Liu Y, Zhang P. Levels of Tc17 cells in thymic epithelial cell tumors associated with autoimmune diseases. Mediastinum 2019;3:8.



