
An overview on the differential diagnostics of tumors of the anterior-superior mediastinum: the pathologist’s perspective
An insight into the pathology of the anterior-superior mediastinum
The anterior-superior mediastinum is affected by a variety of tumors, which mainly derive from its main cellular components, the thymus being the only organ solely present in that area, together with lymph nodes of the anterior mediastinum. Ectopic tissues and embryonic remnants are also found, which could give rise to tumors and to endocrine dysfunction. According to Takeda et al. (1), the most common location of neoplasia in the mediastinum is the anterior compartment (68%) in adults, whereas the posterior mediastinum is more frequently affected in children (52%). Neoplasias of pediatric and adult age show different demographical, clinical and histological spectrum (2). In the so-called “prevascular area” of the mediastinum, a variety of tumors derives from stroma, vascular and neural elements. These tissues accompany the parenchymal cells of the thymus. In the review of Carter et al., in this issue (3), the main imaging features of the thymus according to age and functional state and neoplastic transformation are discussed. The occurrence of benign and malignant mediastinal masses and of inflammatory/infectious diseases adds complexity to the diagnostic process of these deep-seated lesions (4).
From the early developmental stages to aging: a survey of thymic cellular components
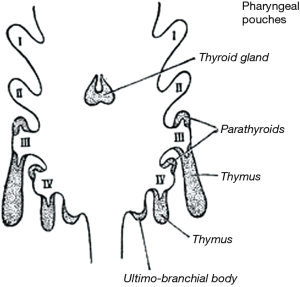
The human thymus originates as a paired anlage from the third pharyngeal pouch endoderm during the early phases of organogenesis (Figure 1), and reaches its final destination in the mediastinum by progressive descent and complex organogenetic events (5-8). However, ectopic thymic tissue may be found along the line of descent as well as in different areas of the mediastinum/thoracic cavity. The papers published by Weissferdt and Moran (9) and by Rashidfarokhi et al. (10) list the main sites where ectopic thymic tissue may occur both from pathological and imaging perspectives. Different types of epithelial cells (ECs) develop in the thymus, and cortical and medullary compartments are established. Several detailed electron microscopic and immunohistochemical studies have demonstrated the heterogeneity of EC (11-13). Medullary TEC (mTEC) and cortical TEC (cTEC) are also functionally heterogeneous (8). In fact, mEC are involved in establishing the central tolerance, and cEC are involved in the positive/negative selection of lymphocytes (14,15). As far as Hassall’s corpuscles (HCs) are concerned, their origin and function remain an enigma. HCs resemble other types of stratified squamous epithelia, although they contain different cell types (16) and are believed to represent the terminally differentiated stage of mTEC (17). The concentration of BCL2 positive B-lymphocytes in HC suggested they play a role in the regulation of lymphopoiesis (16). Moreover, the perivascular space (PVS), which constitutes a specific and significant compartment of thymic tissue, develops within the thymic capsule but outside the EC network (18).
Regarding the origin of T lymphocytes in the thymus
According to von Gaudecker and Müller-Hermelink (19), by the 9th week of gestation, prethymic lymphoid precursor cells, derived from the bone marrow (BM), begin to invade the thymus anlage. There, they finally mature to committed post-thymic T cells through a very complex process (20). The T-cell lineage committed BM progenitors enter the thymus via veins in the cortical tissue close to the corticomedullary junction, and then they migrate into the thymic tissue. A specific T-cell transcriptional program is acquired by the T-cell progenitors. Furthermore, specific cell surface antigens are lost and/or acquired. cTEC play a role in the positive selection of lymphocytes, and drive the maturation of T lymphocytes whereas mTEC, through the promiscuous expression of tissue-restricted antigens (TRAs) and direct presentation of these TRAs, play a role as a self-antigen reservoir and as antigen-presenting cells (APCs) (21,22). However, a continuous “cross-talk” is necessary among epithelial and lymphoid components in order to achieve a normal thymic development (23). Thymopoiesis continues to occur in the thymus of human adults late in life despite the age-related thymic involution (18).
Regarding the origin of B lymphocytes in the thymus
The precise B lymphopoiesis which occurs in the thymus has yet to be completely clarified. However, it appears that in the thymus both intrathymic and conventional B lymphopoiesis occurs. B cells reside in the thymic medulla, specifically at the cortico-medullary junction (24). Thymic B cells were first identified in 1987 in human formalin-fixed, paraffin embedded (FFPE) tissue sections (25). B cells can be seen in the Thymus in early fetal life and show a distinctive phenotype in comparison to other B cell subsets (26), given that it appears they develop independently from BM precursors, and that there is an intrathymic independent pathway for B cell development (14,24). Thymic B cells display unique tolerogenic features and an unclear relationship regarding peripheral B cells (14). Moreover, along with mTEC, they express the autoimmune regulator (AIRE) gene, a transcription factor that controls the negative selection of self- reactive T cells and the complex T-cell development (27). Two distinct B cell populations were also described in thymic epithelial tumors (TETs), both in extra-epithelial (in the PVS) and in intra epithelial compartments (28).
Accessory cells of the thymus
In addition to the main immune cell types, the thymus contains classical dendritic cells (cDCs) of intrathymically and peripherally derived subsets, with antigen presenting cell (APC) capability (29). Moreover stromal cells such as macrophages, which have a distinctive role in the removal of apoptotic lymphocytes, can also be found (30). Plasmacytoid DC with a presumptive role in presenting peripherally acquired antigens for central tolerance induction and interdigitating dendritic cells (IDCs) are also present in the thymus (29,31,32). However, thymic dendritic cells subsets and functions are still ill-defined (33).
The PVS
The PVS constitute an interface between the central thymus and the periphery. In the PVS, both peripheral T and B cells occur, similar to lymph nodes (18,26). The T lymphocytes have a phenotype of cytotoxic or memory T cells, so evidently they immigrate from the periphery. A subset of B cells in the PVS derives from germinal centers. In fact, they express somatic mutations in immunoglobulin genes (18,34).Thymic B cells from the thymic medulla or PVS could give rise to B cell thymic lymphoma (35).
On the supposed origin of germ cells in the thymus
Along the midline, germ cell tumors (GCTs) also develop, the anterior mediastinum constituting the main site of extragenital GCT development, alongside with retroperitoneum. It has been suggested that during embryogenesis ectopic germ cells diffuse in several sites including thymus (36-38) and that these cells could give rise to GCT (39). Benign teratomas could arise from cells derived from the branchial clefts (3rd and 4th) which give origin to the thymic gland according to some studies. As referred from Busch et al. (40) extragonadal GCT could also derive from germ cells that have spread throughout the body during embryogenesis to contribute to important regulatory, hematological or immunological processes.
Mediastinal GCT and their differential diagnoses have been extensively discussed by Kalhor and Moran in a paper of this issue (41) and will not be discussed further here.
All the above-mentioned cell types may develop tumours. However, age and clinical/imaging contexts vary greatly and, in this paper, we propose some examples of difficult or paradigmatic diagnoses.
Diagnostic approach to anterior mediastinal tumors
Section 1: the main epithelial and lymphoid tumor types
TETs
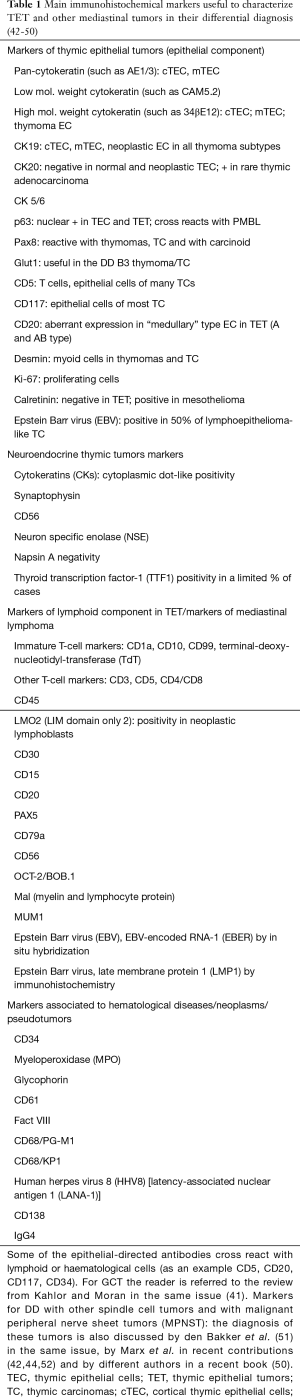
TETs are a distinctive group of rare tumors with unique properties. The WHO classification (42), in widespread use, lists EC tumors with organotypic and morphologic features and immunological properties, the thymoma (THY) (Figure 2), whereas thymic carcinoma (TC) do not show organotypic and functional properties of differentiated EC. TC of different subtypes reproduce specific morphologic features of carcinomas that arise in other organs. However, some specific immunophenotypic features can assist in the pathological diagnosis. Moreover, the WHO classification includes among TET the neuroendocrine tumors. Table 1 lists the main immunohistochemical markers useful to characterize TET and other mediastinal tumors.
Full table
Thymoma and TC
Several classification schema developed in the 20th century, reported and discussed (53) in this issue. In recent years, the World Health Classification (WHO) (54) proposed a compromise following a long debate among pathologists from several countries. In this setting, thymoma was defined as deriving or from the “atrophic”, effete EC of adult thymus, or from the “bioactive” EC of the young thymus. With time, this view provided the basis for “classifying” thymoma. According to the schema proposed by Moran and Suster, however, thymoma could be classified according to the presence of atypia in EC (55,56). In a recent paper (57), cases from different sources were classified according to this schema and staged according a recently proposed system (58); the authors provided outcome data supporting their view.
Thymoma
In the WHO classification, both in the 2004 (59) and 2015 (42) editions, the morphology, immunoarchitecture, imaging and clinical findings of mediastinal masses were integrated in THY diagnosis. Furthermore, in the review by Marx and co-workers in this issue (43), the pathology of myasthenia gravis (MG) associated thymoma is explained in detail; a description of the WHO histotypes and principles of differential diagnosis (DD) is reported. MG is usually associated with the AB or B type thymoma, i.e., the THY with a cortical component. Filosso et al. (60) reported a positive correlation between MG and B-component containing THY. Weis et al. (61), in a series of 4,221 thymoma from the International Thymic Malignancy Interest group (ITMIG) database (62), reported that MG is more frequent in type B1–3 thymomas (35–49%) than in type A and AB (25–26%).
The TC
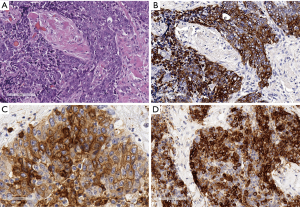
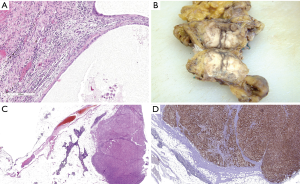
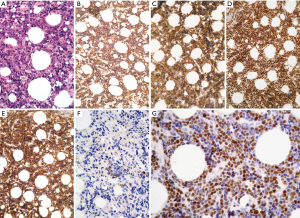
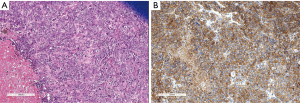
TC of different subtypes such as the ones listed in the WHO classification (42) occurs in the anterior superior mediastinum, the squamous cell carcinoma (SQCC) being the most frequent among the several rare subtypes (Figure 3A,B). TCs also rarely develop in thymoma (63), requiring specific attention and reporting rules, such as discussed by Marx et al. (44). The micronodular TC and the malignant counterpart of the micronodular thymoma (64,65) merits attention here due to the rarity of this histotype, which is yet to be listed in the WHO classification. Relevant immunohistochemical markers can support diagnosis because the expression of CD5, CD117 is useful in distinguishing TC from carcinomas that arise in other organs (45,46,66-72) (Figure 3C,D), whereas GLUT1 and MUC1 appears to be relevant regarding the DD of TC with B3 thymoma (45,73,74). PAX8, a transcription factor involved in the embryonic development of a limited number of organs, and found to be expressed in TET (47,48) and a few other tumor types (75,76), should also be considered in DD with other non thymic tumors.
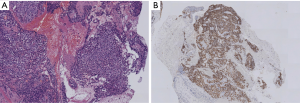
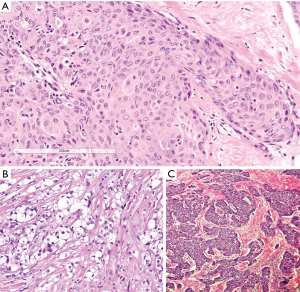
Thymic adenocarcinomas have also been shown to react with CD5 and CD117 (77). Mucinous and enteric types of thymic adenocarcinoma have been described (78,79) and have been frequently associated to thymic cysts. The well-known occurrence of cystic tumors in the mediastinum (80) points to the need of an adequate sampling of every removed cystic lesion of the mediastinum (81,82). Magnetic resonance imaging (MRI) had been described as a powerful tool to distinguish benign cysts from mediastinal neoplasms (83,84). Nuclear protein of the testis (NUT) midline carcinoma [or lethal midline carcinoma, or TC with t(15;19) translocation], also included among TC, is a highly malignant undifferentiated type of carcinoma occurring at times in the thymus (85,86), but also in the lung (Figure 4A). This diagnosis requires a specific immunohistochemical (87) (Figure 4B) and fluorescence in situ hybridization (FISH)-approach in order to be diagnosed (88). In this paper, we present examples of a thymic well differentiated SQCC (Figure 5A) (89), an example of clear cell carcinoma (Figure 5B) (90), and of an “anaplastic” carcinoma (91) metastatic to the liver (Figure 5C).
Neuroendocrine thymic tumors (tNETs)
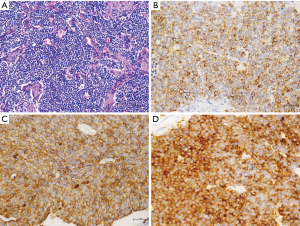
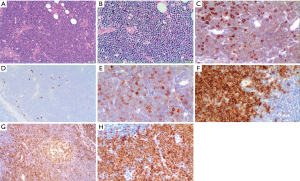
In the thymus, the very rare occurrence of primary neuroendocrine tumors (tNETs) is reported, and their nomenclature and subtyping follow the general rules applied also for neuroendocrine lung tumors (42). A recent review reports an update of morphological features, immunohistochemical staining and reporting rules in combined cases (92). The well differentiated form of tNET, the carcinoid tumors of the thymus, also arises in the framework of MEN type 1. An association with smoking was reported (93) and tNET accounts for almost 20% of multiple endocrine neoplasia type 1 (MEN1)-associated mortality (94). Immunohistochemical markers of tNET include those currently utilized for lung neuroendocrine tumors, whereas genetic markers appear to be different (95). Figure 6 shows the main morphological and immunohistochemical features of a case of atypical thymic carcinoid. Moreover, PAX8 may support the DD of tNET according to the contexts, but antibody clone and clinical information should also be considered (96,97).
Primary lymphoma in the anterior superior mediastinum
Lymphomas in the mediastinum account for 15% of mediastinal masses; they arise either in the thymus or from the mediastinal lymph nodes and only about 10% are primary (98). In biopsies performed on a lymphoid mass, it is difficult to find thymic remnants and other morphological findings that could indicate a thymic origin due to the fact the neoplastic growth has a destructive action. However, when these remnants are seen they should be correctly recognized as such. We will focus here on the specific lymphomas arising or considered to derive from the thymus itself.
Due to the intrathymic presence of an immature T cell compartment as well as to the existence of peripherally derived B and T cells and of a specific intrathymic B-lymphopoiesis, it is not surprising that both lymphoma of immature precursor type (T and B) (99,100) as well as cases of peripheral T and B-cell lymphoma occur in the thymus. Hodgkin lymphoma of classic type (cHL) of thymic origin represents the most frequent mediastinal lymphoma (101). Among the lymphoma occurring in the mediastinum, T cell lymphomas of precursor type predominate in pediatric age, whereas, in the young adult and in adult age, the most frequent lymphomas are of B cell origin or are cHL of the thymus (102-104).
The clinical presentation is usually an important part of the diagnostic process because T-lymphoblastic lymphomas (T-Lb lymphomas) arise, and are usually associated with T-cell lymphocytosis, in pediatric age or in a younger age group, by comparison with TET. cHL of the thymus is predominantly a tumor of the young age and primarily of females. B-cell lymphoma of primary mediastinal B cell type (PMBL), which primarily arises in the thymus, usually shows an acute or sub-acute presentation, with symptoms that include fast growing mass and respiratory difficulties, the female sex and young or young-adult ages being predominantly affected (105,106). Moreover, among the tumors of mature B lymphocytes, the mucosa associated lymphoid tissue lymphoma (MALT lymphoma) of the thymus should be also considered (107), mostly when occurring in patients affected by autoimmune diseases (108,109). We present here in detail the prevailing lymphoma types in the Mediastinum, and we provide examples of neoplasias that derive from the accessory cells of the thymic lymphatic tissue.
cHL
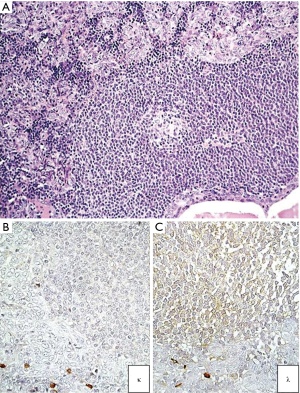
The most frequent subtype of cHL in the mediastinum is the nodular sclerosis (NS) variant (110), constituting about 50–70% of primary mediastinal lymphoma (cHL-NS). It arises from a thymic B cell (111). The diagnosis relies on the demonstration of typical C30+ cells, which are often very rare, in a mixed fibroinflammatory background (112) (Figure 7A,B). Regarding the thymus, the cHL or infiltrates it or it is sharply demarcated from the thymus. Moreover, in cHL reactive EC proliferation and/or cystic changes (Figure 7C) can be elicited, simulating a thymoma. Therefore, again we suggest sampling every mediastinal cystic lesion extensively because foci of HL could be found in the wall (110). The lymphoma usually forms large firm masses with foci of necrosis and eosinophilic abscesses. cHL frequently constitutes a differential diagnostic problem with the primary mediastinal B cell lymphoma (PMBL), which is also associated to sclerosis. In fact, cHL-NS and PMBL shows the same (B) cell origin, similar morphology and may show the similar clinical presentation. Furthermore, some immunophenotypical and gene expression profile similarities exist (113). In Table 2, a list of the main immunophenotypical characteristics of neoplastic lymphoid cells in HL, in PMBL and in the entity denominated “Gray Zone Lymphoma” (GZL) (118) (B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and classical Hodgkin lymphoma in the 2017 WHO classification) (49) is provided.

Full table
PMBL
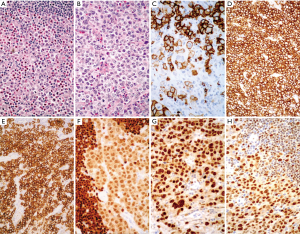
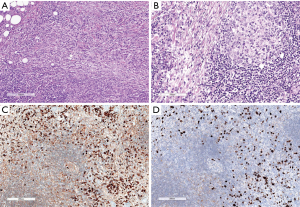
This lymphoma was first described by Menestrina et al. (119) and named mediastinal B cell lymphoma with sclerosis. In fact, a distinctive fibrosis is associated, with large and medium-large pleomorphic cells, with clear cytoplasm in the central part and peripherally distributed lymphocytes in the sclerotic background (Figure 8A,B). This lymphoma usually forms bulky, solid masses >10 cm, with local symptoms of rapid growth, invasion and compression of vital structures. The tumor is limited to the thorax with no involvement of lymph nodes or other lymphoid organs (only supraclavicular nodes are eventually reached). Neoplastic infiltrating CD20+ B cells (Figure 8C) have pleomorphic nuclei, ranging from regular, round nuclei to irregular, multilobulated forms. The cytoplasm is pale to clear. In certain cases, the neoplastic cells are large with prominent eosinophilic nucleoli, which resemble Reed-Sternberg cells or variants. CD30 is weakly or focally expressed (Figure 8D), but MAL, a protein involved in lymphocyte signal transduction, is expressed (120) by PMBL, and the inflammatory background is absent. The MAL antibody is directed against a membrane protein and targets a minor subpopulation of thymic medullary B cells (121). Specific genetic features are exhibited by the PMBL such as gains in loci of 9p24 and 2p15 as well as Xp11.4-21 and Xq24-26 (122,123).
GZL
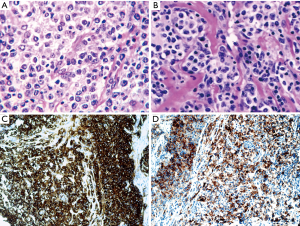
GZL or “B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and classical Hodgkin lymphoma” according to the 2017 WHO classification (49) is a lymphoma with morphological (Figure 9A,B) and immunohistochemical characteristics (Figure 9C,D,E,F,G,H) intermediate between HL and PMBL (124). The pathological tissue often contains “lacunar” cell-forming aggregates, in the subtype known as “syncytial variant”. In the case showed here neoplastic cells were coexpressing CD20, CD30, PAX5 and OCT2. GZL originates in the Mediastinum from a thymic medullary B cell (118). It arises in young patients (20 to 40 years) with a large anterior mediastinal mass eventually involving the supraclavicular lymph nodes. The genetical and epigenetic alterations reported show similarities and differences with those reported for PMBL and HL (125,126). The diagnostic criteria were recently refined in a retrospective large series from Sarkozy et al, in the framework of the lymphoma study association (LYSA) (115) and in the recently published WHO classification of haematological malignancies (49). A diagnostic scoring system was also proposed (116). We previously listed in Table 2 the main differential diagnostic characteristics of these lymphoma types (114,115,117). The adequate diagnosis is crucial due to clinical differences and treatment decisions (127,128).
MALT lymphoma of the thymus
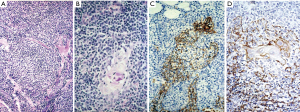
Mature, peripheral B-cell lymphomas also occur in the thymus, the most common being the mucosa-associated B cell lymphoma of MALT type (MALT-LYM) (129). MALT-LYM of the thymus is often cystic. This rare lymphoma type usually develops in autoimmune diseases (130-133) and is characterized by scant residual thymic EC network, and by lymphoepithelial (LE) lesions involving HC (Figure 10). The biological substrate of autoimmune disease could stimulate the formation of acquired MALT (107,134).
Neoplasms of accessory cells of the lymphatic tissue
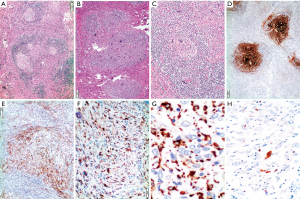
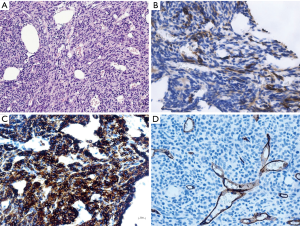
This group of rare neoplasias includes those deriving from Langerhans cells (sarcoma and Langherans cell histiocytosis), histiocytic sarcoma, Follicular dendritic cell sarcoma, and interdigitating reticulum cell (IDC) sarcoma (42). These tumors as a group should be differentiated from the other sarcoma/mesenchymal tumors, which occur in the mediastinum, reviewed and discussed by den Bakker et al. in the same issue (51). Moreover, it should be pointed out that Langerhans cell histiocytosis often occurs in children, leading to widespread involvement, whereas in adults, thymic involvement is often marginal, and limited to small aggregates of LC in otherwise thymic hyperplasia or in a thymic cyst (135). Accessory cell sarcomas have been rarely reported to develop in GCT of the mediastinum (42). The follicular dendritic cell sarcomas (FDCS) often arise in the framework of Castleman disease (CD). CD is a heterogeneous group of non-neoplastic lymphoproliferative disorders of the lymphatic tissue, occasionally giving rise to neoplasms of the constituting cells (both lymphoid and “accessory”) (136-138) (Figure 11). In the mediastinum, the most frequent type of CD is the Hyaline-vascular type (HV), but a plasmacellular type (PC) is also described and usually associated to multicentric disease (139-141). Most of the CD of multicentric types are associated with polyclonal hypergammablobulinemia, elevated VES rate, increase in LDH or IL-6 or with trombocytopenia; moreover multicentric CD is often associated with HHV-8 and HIV infections (142,143).
Some of the neoplasias described may simulate sclerosing (or fibrosing) mediastinitis (SM), which will be discussed later.
Other tumors of the thymus, such as GCT and soft tissue tumors have been presented by other authors in this issue (41,51) and will not be discussed here.
Metastasis in thymus/mediastinum
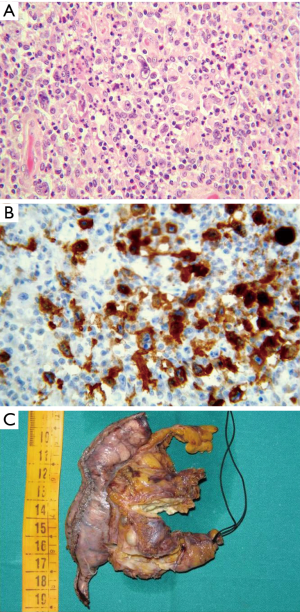
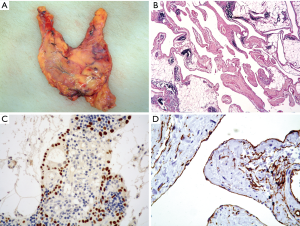
The incidence of thymic metastases is difficult to establish due to the possibility of secondary thymic involvement by diseases which first involve the mediastinal lymph nodes. This statement was first made by Castleman (144). Middleton (145) first performed a detailed autoptic study of the thymus in patients that had deceased due to multiple pathologies. He described the patterns of involvement of the thymus in several cases. According to Suster and Moran, the metastases constitute the prevailing neoplastic pathology in the mediastinum (146). Usually the metastases involve the middle mediastinum or hilar lymph nodes. Teratoma metastasis from the testis also occurs in the mediastinal lymph nodes (Figure 12A). Metastatic lung carcinomas involve the mediastinal lymph nodes more frequently than other tumors. Melanoma metastasis is also frequently reported in the thymus (Figure 12B,C,D). However, also tumors from the breast could involve the thymus. We personally had the occasion to study a breast cancer metastasizing in a thymoma, likely through the lymphatic drainage (not shown here). Moreover, thyroid carcinoma metastases are reported as being more frequently localized to the upper mediastinum (147,148). Immunohistochemistry plays a relevant role in the identification or confirmation of the primary site (42,46,72).
Section 2: practical approach to the DD of lesions of the anterior mediastinum
A variety of different diagnostic/surgical approaches are adopted by clinicians when facing a mediastinal mass of unknown origin. The clinical examination should include an immunological check-up including anti-acetylcholine receptors and anti-nuclear antibodies, together with a complete examination of blood cells and serum protein electrophoresis. The imaging, the clinical setting of TET and of the main different neoplasms occurring in the mediastinum have been reported extensively by Carter et al. (3,106) and informative studies have also been published by den Bakker et al. (38), by Marchevsky et al. (105) and in recently published books/book chapters (50,52), so we will not report on them here in detail. The work-up of tumors which appear to be resectable and have the radiologic appearance of TET, such as a distinct lobulated architecture, does not include a diagnostic pre-treatment biopsy (149).
In well fixed and stained cases, the diagnosis of TET, as also confirmed in the WHO classification (42) can usually be carried out on H&E stains of well-sampled surgical cases. The sampling of tumors must include at least one paraffin block every centimeter size (150). The use of immunohistochemistry in the routine setting is first of all needed in the evaluation of surgical biopsies and of core biopsies (105). These specimens must be evaluated in an expert center because an experienced pathologist in this particular type of tumor/anatomical area is recommended. The hematolymphoid diagnostic experience has also been recommended because, as previously described, TET and lymphomas or other haematological diseases often require DD and/or the awareness of rare diseases is needed. A panel of immunohistochemical stains is suggested as mediastinal masses require interpretation of patterns, recognition of normal remnants of background structures and awareness of the variety of neoplastic/pseudoneoplastic patterns (38,70,151). The previously reported Table 1 reports a list of antibodies which are useful in the DD of TET from other primary malignancies and from metastases (45,46,48,69,72,73,152-156).
A contribution of cytology in the DD of thymic tumor remains controversial (157). Certainly, fine needle aspiration (FNA) may reveal ECs in an immature T-cell component, thus providing elements for a diagnosis of thymoma. However, the thymoma diagnosis and subtyping should be performed on histological specimens, due to the complexity and variety of cellular patterns. In fact FNA and also core biopsy provide scant material (158). When immunohistochemistry can be performed, an antibody panel should be considered given that the use of single markers could be misleading (38,105).
In this overview, our aim is to present first examples of (I) lymphoid-cell-rich lesions of T-cell type; (II) lymphoid-cell-rich lesions of B-cell type; (III) the spectrum of CD34+ neoplasias in mediastinum (or rare haematological localizations and/or vascular tumors); (IV) lymphocyte poor, fibrosing lesions with different pathogenesis and biological meaning; (V) solid or vascular tumors with overlapping/aberrant immunophenotypical features. It should be taken into account that the diagnosis of lymphoma in Europe is largely based on histological and immunohistological findings, whereas, in the United States, the cytofluorimetric approach is generally used for lesions suspected to be lymphoma. Therefore, we show our routine experience in cases which could represent a difficult DD.
The spectrum of lymphoid-cell-rich lesions of T-cell type
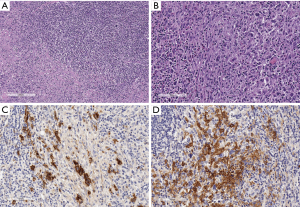
Most lymphocyte-rich lesions in the anterior mediastinum contain T-cells, with immature phenotypes predominating. Both B1 thymoma and T-lymphoblastic (T-Lb) lymphoma may be similar in appearance at first glance, and both tumors in addition are frequently necrotic. Demographical data and clinical presentation are usually of support in the diagnosis, so underlying—once again—the need for continuous clinico-pathological correlation. Figure 13A shows a core biopsy of a B1 thymoma. In the area shown, the high lymphocytic content almost obscures the epithelial network. However, at higher magnification (Figure 13B) and by the cytokeratin staining (Figure 13C), the EC network is highlighted, at the periphery of the lymphoid areas. The figure shows that CD3+ lymphocytes (Figure 13D) in B1 thymoma are also Tdt positive (Figure 13E) but LMO2 negative (Figure 13F). Otherwise, the routine immunophenotypical features of T-lymphocytes overlap in the 2 neoplasias, B1 thymoma and T-Lb lymphoma, as shown in Figure 14. Please note, in addition, that in T-Lb lymphoma exceptionally EC framework remnants could still be present—such as thymic or cystic epithelial structures. Therefore, the evaluation of the structure formed is equally important as the EC network detection, and the under-evaluation of these different characters may constitute a pitfall for the diagnosis. Furthermore, the morphological features of lymphocytes shown in the two cases are very different, as in T-Lb lymphoma the T-lymphoblasts are highly atypical (Figure 14A,B). The neoplastic T lymphoblasts were also positive for CD3 (week), CD1a, CD10, Tdt (Figure 14C,E,F,H) and negative for PAX5 (Figure 14D). Phenotypic refinement is of further support: a recently available antibody, LMO2, proved to be useful in the characterization of lymphoblast-containing proliferations in the mediastinum (159). In fact, this antibody (Ab) does not stain normal lymphoblasts in the thymus but only neoplastic lymphoblasts (160) (compare Figure 13F of B1 Thymoma with Figure 14G of T-Lb lymphoma). Similar findings were reported with NOTCH1 intracellular domain immunohistochemistry, positive with T-lymphoblastic leukemia cells and negative in lymphocyte-rich thymoma (161). In this case, the clinical/demographical setting is also very different, as T-Lb lymphoma is predominantly if not always a tumor of childhood or young age and is associated to or develops a lymphocytosis.
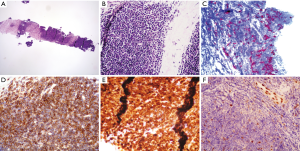
There are further difficulties in evaluating immature T-cell containing mediastinal lesions. In fact, it should be mentioned that thymoma could be associated with T-cell lymphocytosis, and that cases of thymoma/TC and T- lymphoblastic lymphoma occurring in the same patient have been recorded (162). In similar cases, T-clonal rearrangement evaluation should be associated with the routine investigations. In Figure 15A,B,C, the main morphological and immunohistochemical features of a B2 type thymoma associated with T-cell lymphocytosis are shown (163). A polyclonal T cell receptor rearrangement was found both in the tumor and in the peripheral blood (PB) (not shown). Polymyositis and myocarditis were also associated (Figure 15D,E,F) (164). The interpretation of these cases requires a careful multidisciplinary approach due to the fact that the clinical picture could also be very severe and complex.
Peripheral T-cell lymphomas have also been reported in the thymus/mediastinal lymph nodes (165), so any possibility should be considered when faced with a T-cell-rich lesion in the mediastinum.
The spectrum of lymphoid-cell-rich lesions of B-cell type
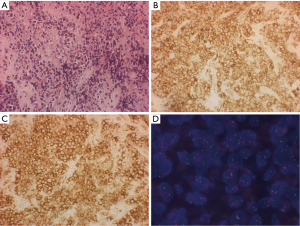
B-lymphoblastic lymphomas (B-Lb), although rare, may affect the mediastinal area/structures, as shown in Figure 16. This tumor occurred as a pericardial mass in a 56-year-old woman (Figure 16A) and the immunohistochemistry was fundamental in order to characterize the specific phenotype of the B Lymphoblasts, positive for CD34, CD10, PAX5 and CD79A (Figure 16B,C,D,E) but negative for CD20 (Figure 16F). The coexpression with CD34 of CD10, CD79a, PAX5 and CD38 (not shown) together made it possible to establish the diagnosis of B-Lb lymphoma even in the absence of CD20 positivity.
B-Lb lymphomas in the Thymus usually occur in the setting of a systemic disease, with BM involvement, but it is peculiar to note that a primary mediastinal mass may also occur (166,167).
Among low-grade B-cell lymphoma, precursor lesions of MALT lymphoma have been described by Moran et al. (168,169). We also previously reported the removal of a thymic mass showing follicular lymphatic hyperplasia with a monoclonal B lymphocyte background (170). Among precursor lesions, the description of the rare occurrence of monoclonal B cell populations/low grade-B cell lymphoma occurring in micronodular thymoma (MNT) was previously carried out (Figure 17). A pathogenetic link between MALT lymphoma of the thymus and a MNT-type proliferation was proposed (171). This topic is still a matter of debate (65) and the description of other cases in the future will provide new insights.
The spectrum of CD34+ neoplasias in mediastinum (or rare haematological localizations and/or vascular tumors)
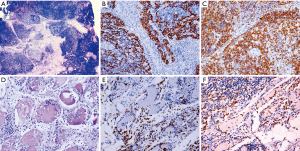
CD34 gp10-120, mucosialin, is a MY10 sialomucin and it is a common marker of vascular tumors as well as of hemopoietic progenitor cells. This antibody can be used in the context of wide panels to characterize tumors of hemopoietic lineage progenitors. Among CD34+ neoplasia in the mediastinum, in fact, we would like now to mention the occurrence of CD34 positivity in blastic haematological neoplasias, such as the tumor-forming myeloid sarcoma (MS) (172) (also named granulocytic sarcoma) arising in the mediastinum de novo (173,174) or in the setting of myeloproliferative diseases as a relapse (175) or as blastic crisis (176). In that field, we show here the case of a man affected by a chronic myeloid leukemia (CML) who developed a mediastinal mass and only subsequently was faced with a blastic crisis. Figure 18 shows the reasons for difficulty in understanding, in a biopsy, the morphological features of cells infiltrating a sclerotic mediastinal stroma (Figure 18A), even with the positivity for CD45 (Figure 18B) and CD34 (Figure 18C) of these cells. Subsequently, the complete history of the patient was referred and the FISH hybridization procedure confirmed the BCR/ABL fusion positivity (Figure 18D) in these blasts arising in CML. It should be mentioned here that extramedullary hematopoiesis (EMH), which usually occurs in a posterior mediastinum localization, has been exceptionally reported in an anterior mediastinal localization (177,178). It should be also remembered here that haematological malignancies may arise also in the framework of GCT of the mediastinum (179,180).
The same marker, CD34, can be used in the context of wide panels either in the study of dubious epithelial rich thymoma of A type in order to exclude mesenchymal tumors such as synovial sarcoma (SS) (181,182), or solitary fibrous tumor (SFT) (183) or in the context of vascular tumors. Mesenchimal tumors and their DD have already been widely discussed by den Bakker et al. in a paper of this issue (51). CD34 staining is of support in the diagnosis of a rare Cystic lymphangioma of the mediastinum (184) (Figure 19), a rare tumor occurring in a 66-year-old man (185). Moreover, the CD34 positivity was the only positive vascular marker (186) in the evaluation of a large mediastinal mass expanding also outside the thorax in the supraclavicular soft tissue (Figure 20A,B). In this case, the high grade cytology and the positivity for the endothelial marker CD34 together with the negativity for several other markers—among them CD31, another fundamental vascular tumor marker—made it possible to identify an epithelioid angiosarcoma in a 26 year- old young male.
The spectrum of fibrosing lesions in the mediastinum
The spectrum of fibrosing tumors/pseudotumors in the mediastinum includes on the one side sclerosing thymoma, described in the WHO classification manual as an epithelial tumor with overwhelming fibrosclerotic stroma (42) but it also includes the cases of HL of the mediastinum. In particular, the NS subtype of cHL is associated with diffuse sclerosis (110) (Figure 7); the same sclerotic background is characteristic of the PMBL, previously denominated Mediastinal large cell lymphoma of B type with sclerosis (119) (Figure 8). In Figure 21, a further case of cHL is shown, with a peculiar increase in IDC, which could simulate an IDC sarcoma. Moreover, the sclerosing mediastinitis (SM), a peculiar disease with immune/autoimmune pathogenesis which rarely affects the mediastinum is known to produce abnormal fibrous tissue in the mediastinum.
SM
IgG4-related disease (IgG4-RD) is an idiopatic fibroinflammatory disorder associated with hypergammaglobulinemia and increased serum levels of IgG, particularly IgG4. The clinical spectrum of the disease includes sclerosing (autoimmune) pancreatitis (AIP), sclerosing cholangitis, retroperitoneal fibrosis (IRF), sclerosing sialadenitis (Küttner tumor), orbital pseudotumor, lymphadenopathy, nephritis, thyroiditis and interstitial pneumonia. All these disease forms lead to inflammatory pseudotumors, which develop in different organ/systems, including mediastinum, with a marked IgG4+ plasma cell infiltrate (187,188). Figure 22 shows a case of IgG4 disease developing in a salivary gland (Küttner tumor) in an old woman. Cellular and storiform fibrosis, lymphoplasmacytic infiltration, increased numbers of IgG4-positive plasma cells and obliterative phlebitis are the hallmark histological features in IgG4-RD. In the mediastinum, lymph node enlargement is associated with lung disease and it is very frequent in patients with AIP (189). SM is an aggressive syndrome characterized by the formation of invasive fibrous tissue which substitutes the adipose tissue with dense sclerosis and eventually compromises vital structures in the mediastinum. SM can be idiopathic or secondary to infections and malignancies. In cases of fibrosing lesions of the mediastinum, occult malignancies should be excluded by extensive examination of the fibrous tissue. In fact, in some cases, SM was masquerading a malignancy, especially lymphoma (165,190), follicular dendritic sarcoma (191) and mesothelioma (192). Two forms of SM have been described, a focal granulomatous form and a diffuse usually non-granulomatous form (193). Histoplasmosis, tuberculosis, aspergillosis, blastomycosis, cryptococcosis and also sarcoidosis can be responsible for granulomatous SM. Instead, non-granulomatous SM is considered an idiopathic reaction to different autoimmune syndromes, to radiation therapy, or to treatment with methysergide or in the setting of Behçet disease (194,195). All the previously mentioned diseases contribute to the wide clinical spectrum of IgG4-related disease (IgG4-RD) (195).
The spectrum of solid spindle or epithelial/epithelioid tumors in the mediastinum
The epithelial/epithelioid tumor spectrum in the anterior-superior mediastinum first includes the EC-rich types of TET. Type A thymoma is characterized by spindled or oval cells, with a fascicular/storiform or solid pattern, or which form glandular/adenoid structures, or pseudorosettes, or which have a pericytomatous pattern. Type A thymoma shows a very scant T-lymphocytic content of the mature type, as described by Marx et al. (44). Cytokeratins, p63/p40, with CD20 co-expression, in our opinion, are the best marker association in order to better identify Type A thymoma in doubtful cases (Figure 23). This TET subtype can often require a DD with other spindle cell tumors of the mediastinum (196), such as the already-mentioned SFT (183) and SS (43,181). Primary TET arisen in the pleura are negative for Calretinin, a feature assisting in the DD with malignant pleural mesothelioma (MPM) (151,153). SS, especially monophasic fibrous SS arising in the mediastinum, can be diagnosed by a panel of markers (197), including the transcriptional corepressor TLE1 (198,199). The diagnosis can be confirmed by the SYT-SSX fusion gene via (72) fluorescence in situ hybridization (200) as reported by den Bakker et al. (51). Atypical type A thymoma, an entity that has yet to be recognized by the WHO, shows in addition signs of atypia and necrosis (201,202). Moreover, type B3 TET needs to be distinguished from B2 TET as well from thymic SQCC. These last topics are largely described in the paper published by Marx et al. in 2014 (44), in the WHO 2015 classification (42), in a recent book (52) and in the same focused issue (43).
The complex chapter of the DD of primary thymic SQCC vs. lung SQCC metastases is yet to be completed because a very specific immunohistochemical panel is difficult to establish. However, the WHO (42) and several papers have discussed the topic and proposed wide immunohistochemical panels in support of a meaningful clinical and pathological diagnosis (48,72).
Conclusions
The stimuli leading to EC proliferation in the thymus vary and not only neoplastic processes are responsible. Our awareness of the biology of EC in the thymus is very scant, regarding both their immunological interplay and reactivity to different types of local inflammation.
The complexity of structure, embryological derivation and functional activities render the thymus and its derived tumors very complex both for their diagnosis and functional interplay with the entire immune system and…beyond. No similar example of involuted organ in humans is responsible for so many interactions and effects in its pathological state. Assessment of the clinical setting and careful attention to all cell types included in a biopsy/tumor specimen should be applied in order to reach an adequate diagnosis.
Acknowledgments
The authors thank Dr. Enzo Gallo for the editorial assistance and Dr. Michael G. Kenyon for his review of the English language.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Mediastinum for the series “Diagnostic Problems in Anterior Mediastinum Lesions” published in Mediastinum. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med.2018.12.01). The series “Diagnostic Problems in Anterior Mediastinum Lesions” was commissioned by the editorial office without any funding or sponsorship. MM serves as the unpaid Guest Editor of this series and an unpaid editorial board member of Mediastinum from May 2017 to Apr 2019. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Takeda S, Miyoshi S, Akashi A, et al. Clinical spectrum of primary mediastinal tumors: a comparison of adult and pediatric populations at a single Japanese institution. J Surg Oncol 2003;83:24-30. [Crossref] [PubMed]
- Liu T, Al-Kzayer LFY, Xie X, et al. Mediastinal lesions across the age spectrum: a clinicopathological comparison between pediatric and adult patients. Oncotarget 2017;8:59845-53. [PubMed]
- Carter BW, Benveniste MF, Marom EM. Diagnostic Approach to the Anterior/Prevascular Mediastinum for Radiologists. Mediastinum 2018; In Press.
- Naeem F, Metzger ML, Arnold SR, et al. Distinguishing Benign Mediastinal Masses from Malignancy in a Histoplasmosis-Endemic Region. J Pediatr 2015;167:409-15. [Crossref] [PubMed]
- von Gaudecker B. The development of the human thymus microenvironment. Curr Top Pathol 1986;75:1-41. [Crossref] [PubMed]
- Kendall MD. Functional anatomy of the thymic microenvironment. J Anat 1991;177:1-29. [PubMed]
- Vaidya HJ, Briones Leon A, Blackburn CC. FOXN1 in thymus organogenesis and development. Eur J Immunol 2016;46:1826-37. [Crossref] [PubMed]
- Farley AM, Morris LX, Vroegindeweij E, et al. Dynamics of thymus organogenesis and colonization in early human development. Development 2013;140:2015-26. [Crossref] [PubMed]
- Weissferdt A, Moran CA. The spectrum of ectopic thymomas. Virchows Arch 2016;469:245-54. [Crossref] [PubMed]
- Rashidfarokhi M, Gupta J, Leytin A, et al. Ectopic Anterior Mediastinal Pathology in the Chest: Radiologic-pathologic Correlation of Unexpected Encounters with the "Terrible Ts". J Clin Imaging Sci 2016;6:49. [Crossref] [PubMed]
- Von Gaudecker B, Kendall MD, Ritter MA. Immuno-electron microscopy of the thymic epithelial microenvironment. Microsc Res Tech 1997;38:237-49. [Crossref] [PubMed]
- Haynes BF. The human thymic microenvironment. Adv Immunol 1984;36:87-142. [Crossref] [PubMed]
- Abramson J, Anderson G. Thymic Epithelial Cells. Annu Rev Immunol 2017;35:85-118. [Crossref] [PubMed]
- Yamano T, Nedjic J, Hinterberger M, et al. Thymic B Cells Are Licensed to Present Self Antigens for Central T Cell Tolerance Induction. Immunity 2015;42:1048-61. [Crossref] [PubMed]
- Rezzani R, Nardo L, Favero G, et al. Thymus and aging: morphological, radiological, and functional overview. Age (Dordr) 2014;36:313-51. [Crossref] [PubMed]
- Mikušová R, Mešťanová V, Polák Š, et al. What do we know about the structure of human thymic Hassall's corpuscles? A histochemical, immunohistochemical, and electron microscopic study. Ann Anat 2017;211:140-8. [Crossref] [PubMed]
- Matsui N, Ohigashi I, Tanaka K, et al. Increased number of Hassall's corpuscles in myasthenia gravis patients with thymic hyperplasia. J Neuroimmunol 2014;269:56-61. [Crossref] [PubMed]
- Hale LP. Histologic and molecular assessment of human thymus. Ann Diagn Pathol 2004;8:50-60. [Crossref] [PubMed]
- von Gaudecker B, Müller-Hermelink HK. Ontogeny and organization of the stationary non-lymphoid cells in the human thymus. Cell Tissue Res 1980;207:287-306. [Crossref] [PubMed]
- Famili F, Wiekmeijer AS, Staal FJ. The development of T cells from stem cells in mice and humans. Future Sci OA 2017;3:FSO186 [Crossref] [PubMed]
- Derbinski J, Schulte A, Kyewski B, et al. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol 2001;2:1032-9. [Crossref] [PubMed]
- Hinterberger M, Aichinger M, Prazeres da Costa O, et al. Autonomous role of medullary thymic epithelial cells in central CD4(+) T cell tolerance. Nat Immunol 2010;11:512-9. [Crossref] [PubMed]
- Chinn IK, Blackburn CC, Manley NR, et al. Changes in primary lymphoid organs with aging. Semin Immunol 2012;24:309-20. [Crossref] [PubMed]
- Perera J, Huang H. The development and function of thymic B cells. Cell Mol Life Sci 2015;72:2657-63. [Crossref] [PubMed]
- Isaacson PG, Norton AJ, Addis BJ. The human thymus contains a novel population of B lymphocytes. Lancet 1987;2:1488-91. [Crossref] [PubMed]
- Fend F, Nachbaur D, Oberwasserlechner F, et al. Phenotype and topography of human thymic B cells. An immunohistologic study. Virchows Arch B Cell Pathol Incl Mol Pathol 1991;60:381-8. [Crossref] [PubMed]
- Cepeda S, Cantu C, Orozco S, et al. Age-Associated Decline in Thymic B Cell Expression of Aire and Aire-Dependent Self-Antigens. Cell Rep 2018;22:1276-87. [Crossref] [PubMed]
- Fend F, Kirchner T, Marx A, et al. B-cells in thymic epithelial tumours. An immunohistochemical analysis of intra- and extraepithelial B-cell compartments. Virchows Arch B Cell Pathol Incl Mol Pathol 1993;63:241-7. [Crossref] [PubMed]
- Wu L, Shortman K. Heterogeneity of thymic dendritic cells. Semin Immunol 2005;17:304-12. [Crossref] [PubMed]
- Nitta T, Suzuki H. Thymic stromal cell subsets for T cell development. Cell Mol Life Sci 2016;73:1021-37. [Crossref] [PubMed]
- Kroger CJ, Spidale NA, Wang B, et al. Thymic Dendritic Cell Subsets Display Distinct Efficiencies and Mechanisms of Intercellular MHC Transfer. J Immunol 2017;198:249-56. [Crossref] [PubMed]
- Bendriss-Vermare N, Barthélémy C, Durand I, et al. Human thymus contains IFN-alpha-producing CD11c(-), myeloid CD11c(+), and mature interdigitating dendritic cells. J Clin Invest 2001;107:835-44. [Crossref] [PubMed]
- Gurka S, Dirks S, Photiadis J, et al. Expression analysis of surface molecules on human thymic dendritic cells with the 10th HLDA Workshop antibody panel. Clin Transl Immunology 2015;4:e47 [Crossref] [PubMed]
- Haynes BF, Hale LP. The human thymus. A chimeric organ comprised of central and peripheral lymphoid components. Immunol Res 1998;18:175-92. [Crossref] [PubMed]
- Flores KG, Li J, Hale LP. B cells in epithelial and perivascular compartments of human adult thymus. Hum Pathol 2001;32:926-34. [Crossref] [PubMed]
- Takeda S, Miyoshi S, Ohta M, et al. Primary germ cell tumors in the mediastinum: a 50-year experience at a single Japanese institution. Cancer 2003;97:367-76. [Crossref] [PubMed]
- Oosterhuis JW, Stoop H, Honecker F, et al. Why human extragonadal germ cell tumours occur in the midline of the body: old concepts, new perspectives. Int J Androl 2007;30:256-63; discussion 263-4. [Crossref] [PubMed]
- den Bakker MA, Oosterhuis JW. Tumours and tumour-like conditions of the thymus other than thymoma; a practical approach. Histopathology 2009;54:69-89. [Crossref] [PubMed]
- FRIEDMAN NB. The comparative morphogenesis of extragenital and gonadal teratoid tumors. Cancer 1951;4:265-76. [Crossref] [PubMed]
- Busch J, Seidel C, Zengerling F. Male Extragonadal Germ Cell Tumors of the Adult. Oncol Res Treat 2016;39:140-4. [Crossref] [PubMed]
- Kalhor N, Moran CA. Primary germ cell tumors of the mediastinum: a review. Mediastinum 2018;2:4. [Crossref]
- Travis WD, Brambilla E, Burke AP, et al. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. 4th ed. ed. World Health Organization Classification of Tumours. Lyon: International Agency for Research on Cancer-IARC-Press, 2015.
- Marx A, Ströbel P, Weis CA. The Pathology of the Thymus in Myasthenia Gravis. Mediastinum 2018;2:66. [Crossref]
- Marx A, Ströbel P, Badve SS, et al. ITMIG consensus statement on the use of the WHO histological classification of thymoma and thymic carcinoma: refined definitions, histological criteria, and reporting. J Thorac Oncol 2014;9:596-611. [Crossref] [PubMed]
- Su XY, Wang WY, Li JN, et al. Immunohistochemical differentiation between type B3 thymomas and thymic squamous cell carcinomas. Int J Clin Exp Pathol 2015;8:5354-62. [PubMed]
- Weissferdt A, Moran CA. Immunohistochemistry in the diagnosis of thymic epithelial neoplasms. Appl Immunohistochem Mol Morphol 2014;22:479-87. [Crossref] [PubMed]
- Weissferdt A, Moran CA. Pax8 expression in thymic epithelial neoplasms: an immunohistochemical analysis. Am J Surg Pathol 2011;35:1305-10. [Crossref] [PubMed]
- Asirvatham JR, Esposito MJ, Bhuiya TA. Role of PAX-8, CD5, and CD117 in distinguishing thymic carcinoma from poorly differentiated lung carcinoma. Appl Immunohistochem Mol Morphol 2014;22:372-6. [Crossref] [PubMed]
- Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid tissues. 4th revised ed. World Health Organization Classification of Tumours, vol 2. Lyon: International Agency for Research on Cancer-IARC Press, 2017.
- Franco R, Zito Marino F, Giordano A, editors. The Mediastinal Mass - A multidisciplinary approach. first ed. Current Clinical Pathology: Humana Press, Springer Nature, 2018.
- den Bakker M, Marx A, Ströbel P. The pathology of mesenchymal tumors of the mediastinum. Mediastinum 2018;2:42. [Crossref]
- Marx A, Weis C, Tzankov A, et al. Thymic Epithelial Tumors and Benign Thymic lesions. In: Roden A, Moreira A. editors. Mediastinal lesions Diagnostic pearls for interpretation of small biopsies and citology. Springer International AG, 2017:87-148.
- Marino M, Roden AC. The evolution of the histopathologic classification of thymic epithelial tumors. Mediastinum 2018;2:9. [Crossref]
- Rosai J, Sobin LH. Histological typing of tumours of the thymus. World Health Organization. International histological classification of tumours. 1999.
- Suster S, Moran CA. Thymoma, atypical thymoma, and thymic carcinoma. A novel conceptual approach to the classification of thymic epithelial neoplasms. Am J Clin Pathol 1999;111:826-33. [Crossref] [PubMed]
- Suster S, Moran CA. Histologic classification of thymoma: the World Health Organization and beyond. Hematol Oncol Clin North Am 2008;22:381-92. [Crossref] [PubMed]
- Weissferdt A, Kalhor N, Bishop JA, et al. THYMOMA: A clinicopathological correlation of 1470 cases. Hum Pathol 2018;73:7-15. [Crossref] [PubMed]
- Moran CA, Walsh G, Suster S, et al. Thymomas II: a clinicopathologic correlation of 250 cases with a proposed staging system with emphasis on pathologic assessment. Am J Clin Pathol 2012;137:451-61. [Crossref] [PubMed]
- Travis WD, Brambilla E, Müller-Hermelink HK, et al. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. World Health Organization Classification of Tumours. Lyon: IARC Press, 2004.
- Filosso PL, Venuta F, Oliaro A, et al. Thymoma and inter-relationships between clinical variables: a multicentre study in 537 patients. Eur J Cardiothorac Surg 2014;45:1020-7. [Crossref] [PubMed]
- Weis CA, Yao X, Deng Y, et al. The impact of thymoma histotype on prognosis in a worldwide database. J Thorac Oncol 2015;10:367-72. [Crossref] [PubMed]
- Huang J, Ahmad U, Antonicelli A, et al. Development of the international thymic malignancy interest group international database: an unprecedented resource for the study of a rare group of tumors. J Thorac Oncol 2014;9:1573-8. [Crossref] [PubMed]
- Suster S, Moran CA. Primary thymic epithelial neoplasms showing combined features of thymoma and thymic carcinoma. A clinicopathologic study of 22 cases. Am J Surg Pathol 1996;20:1469-80. [Crossref] [PubMed]
- Weissferdt A, Moran CA. Micronodular thymic carcinoma with lymphoid hyperplasia: a clinicopathological and immunohistochemical study of five cases. Mod Pathol 2012;25:993-9. [Crossref] [PubMed]
- Mneimneh WS, Gökmen-Polar Y, Kesler KA, et al. Micronodular thymic neoplasms: case series and literature review with emphasis on the spectrum of differentiation. Mod Pathol 2015;28:1415-27. [Crossref] [PubMed]
- Hishima T, Fukayama M, Fujisawa M, et al. CD5 expression in thymic carcinoma. Am J Pathol 1994;145:268-75. [PubMed]
- Berezowski K, Grimes MM, Gal A, et al. CD5 immunoreactivity of epithelial cells in thymic carcinoma and CASTLE using paraffin-embedded tissue. Am J Clin Pathol 1996;106:483-6. [Crossref] [PubMed]
- Tateyama H, Eimoto T, Tada T, et al. Immunoreactivity of a new CD5 antibody with normal epithelium and malignant tumors including thymic carcinoma. Am J Clin Pathol 1999;111:235-40. [Crossref] [PubMed]
- Henley JD, Cummings OW, Loehrer PJ. Tyrosine kinase receptor expression in thymomas. J Cancer Res Clin Oncol 2004;130:222-4. [Crossref] [PubMed]
- Pan CC, Chen PC, Chiang H. KIT (CD117) is frequently overexpressed in thymic carcinomas but is absent in thymomas. J Pathol 2004;202:375-81. [Crossref] [PubMed]
- Ströbel P, Hartmann M, Jakob A, et al. Thymic carcinoma with overexpression of mutated KIT and the response to imatinib. N Engl J Med 2004;350:2625-6. [Crossref] [PubMed]
- Zhang K, Deng H, Cagle PT. Utility of immunohistochemistry in the diagnosis of pleuropulmonary and mediastinal cancers: a review and update. Arch Pathol Lab Med 2014;138:1611-28. [Crossref] [PubMed]
- Kojika M, Ishii G, Yoshida J, et al. Immunohistochemical differential diagnosis between thymic carcinoma and type B3 thymoma: diagnostic utility of hypoxic marker, GLUT-1, in thymic epithelial neoplasms. Mod Pathol 2009;22:1341-50. [Crossref] [PubMed]
- Kaira K, Endo M, Abe M, et al. Biologic correlation of 2-[18F]-fluoro-2-deoxy-D-glucose uptake on positron emission tomography in thymic epithelial tumors. J Clin Oncol 2010;28:3746-53. [Crossref] [PubMed]
- Ordóñez NG. Value of PAX 8 immunostaining in tumor diagnosis: a review and update. Adv Anat Pathol 2012;19:140-51. [Crossref] [PubMed]
- Laury AR, Perets R, Piao H, et al. A comprehensive analysis of PAX8 expression in human epithelial tumors. Am J Surg Pathol 2011;35:816-26. [Crossref] [PubMed]
- Kalhor N, Moran CA. Primary thymic adenocarcinomas: a clinicopathological and Immunohistochemical study of 16 cases with emphasis on the morphological spectrum of differentiation. Hum Pathol 2018;74:73-82. [Crossref] [PubMed]
- Maeda D, Ota S, Ikeda S, et al. Mucinous adenocarcinoma of the thymus: a distinct variant of thymic carcinoma. Lung Cancer 2009;64:22-7. [Crossref] [PubMed]
- Moser B, Schiefer AI, Janik S, et al. Adenocarcinoma of the thymus, enteric type: report of 2 cases, and proposal for a novel subtype of thymic carcinoma. Am J Surg Pathol 2015;39:541-8. [Crossref] [PubMed]
- Weissferdt A, Moran CA. Thymic carcinoma associated with multilocular thymic cyst: a clinicopathologic study of 7 cases. Am J Surg Pathol 2011;35:1074-9. [Crossref] [PubMed]
- Jeung MY, Gasser B, Gangi A, et al. Imaging of cystic masses of the mediastinum. Radiographics 2002;22:S79-93. [Crossref] [PubMed]
- Vargas D, Suby-Long T, Restrepo CS. Cystic Lesions of the Mediastinum. Semin Ultrasound CT MR 2016;37:212-22. [Crossref] [PubMed]
- Ackman JB. MR Imaging of Mediastinal Masses. Magn Reson Imaging Clin N Am 2015;23:141-64. [Crossref] [PubMed]
- Madan R, Ratanaprasatporn L, Carter BW, et al. Cystic mediastinal masses and the role of MRI. Clin Imaging 2017;50:68-77. [Crossref] [PubMed]
- Bauer DE, Mitchell CM, Strait KM, et al. Clinicopathologic features and long-term outcomes of NUT midline carcinoma. Clin Cancer Res 2012;18:5773-9. [Crossref] [PubMed]
- Evans AG, French CA, Cameron MJ, et al. Pathologic characteristics of NUT midline carcinoma arising in the mediastinum. Am J Surg Pathol 2012;36:1222-7. [Crossref] [PubMed]
- Haack H, Johnson LA, Fry CJ, et al. Diagnosis of NUT midline carcinoma using a NUT-specific monoclonal antibody. Am J Surg Pathol 2009;33:984-91. [Crossref] [PubMed]
- French CA, Kutok JL, Faquin WC, et al. Midline carcinoma of children and young adults with NUT rearrangement. J Clin Oncol 2004;22:4135-9. [Crossref] [PubMed]
- Suster S, Rosai J. Thymic carcinoma. A clinicopathologic study of 60 cases. Cancer 1991;67:1025-32. [Crossref] [PubMed]
- Snover DC, Levine GD, Rosai J. Thymic carcinoma. Five distinctive histological variants. Am J Surg Pathol 1982;6:451-70. [Crossref] [PubMed]
- Weissferdt A, Moran CA. Anaplastic thymic carcinoma: a clinicopathologic and immunohistochemical study of 6 cases. Hum Pathol 2012;43:874-7. [Crossref] [PubMed]
- Bohnenberger H, Dinter H, König A, et al. Neuroendocrine tumors of the thymus and mediastinum. J Thorac Dis 2017;9:S1448-57. [Crossref] [PubMed]
- Christakis I, Qiu W, Silva Figueroa AM, et al. Clinical Features, Treatments, and Outcomes of Patients with Thymic Carcinoids and Multiple Endocrine Neoplasia Type 1 Syndrome at MD Anderson Cancer Center. Horm Cancer 2016;7:279-87. [Crossref] [PubMed]
- Ye L, Wang W, Ospina NS, et al. Clinical features and prognosis of thymic neuroendocrine tumours associated with multiple endocrine neoplasia type 1: A single-centre study, systematic review and meta-analysis. Clin Endocrinol (Oxf) 2017;87:706-16. [Crossref] [PubMed]
- Ströbel P, Zettl A, Shilo K, et al. Tumor genetics and survival of thymic neuroendocrine neoplasms: a multi-institutional clinicopathologic study. Genes Chromosomes Cancer 2014;53:738-49. [Crossref] [PubMed]
- Liau JY, Tsai JH, Jeng YM, et al. The Diagnostic Utility of PAX8 for Neuroendocrine Tumors: An Immunohistochemical Reappraisal. Appl Immunohistochem Mol Morphol 2016;24:57-63. [Crossref] [PubMed]
- Weissferdt A, Tang X, Wistuba II, et al. Comparative immunohistochemical analysis of pulmonary and thymic neuroendocrine carcinomas using PAX8 and TTF-1. Mod Pathol 2013;26:1554-60. [Crossref] [PubMed]
- Duwe BV, Sterman DH, Musani AI. Tumors of the mediastinum. Chest 2005;128:2893-909. [Crossref] [PubMed]
- Pan Y, Li GD, Liu WP, et al. Lymphoblastic lymphoma and acute lymphoblastic leukemia: a clinicopathologic, immunophenotypic and prognostic study in 153 Chinese patients. Zhonghua Bing Li Xue Za Zhi 2009;38:810-5. [PubMed]
- Bassan R, Maino E, Cortelazzo S. Lymphoblastic lymphoma: an updated review on biology, diagnosis, and treatment. Eur J Haematol 2016;96:447-60. [Crossref] [PubMed]
- Rauthe S, Rosenwald A. Mediastinal lymphomas. Pathologe 2016;37:457-64. [Crossref] [PubMed]
- Laurent C, Do C, Gourraud PA, et al. Prevalence of Common Non-Hodgkin Lymphomas and Subtypes of Hodgkin Lymphoma by Nodal Site of Involvement: A Systematic Retrospective Review of 938 Cases. Medicine (Baltimore) 2015;94:e987 [Crossref] [PubMed]
- Maeshima AM, Taniguchi H, Suzuki T, et al. Distribution of malignant lymphomas in the anterior mediastinum: a single-institution study of 76 cases in Japan, 1997-2016. Int J Hematol 2017;106:675-80. [Crossref] [PubMed]
- Chen L, Wang M, Fan H, et al. Comparison of pediatric and adult lymphomas involving the mediastinum characterized by distinctive clinicopathological and radiological features. Sci Rep 2017;7:2577. [Crossref] [PubMed]
- Marchevsky A, Marx A, Ströbel P, et al. Policies and reporting guidelines for small biopsy specimens of mediastinal masses. J Thorac Oncol 2011;6:S1724-9. [Crossref] [PubMed]
- Carter BW, Marom EM, Detterbeck FC. Approaching the patient with an anterior mediastinal mass: a guide for clinicians. J Thorac Oncol 2014;9:S102-9. [Crossref] [PubMed]
- Isaacson PG, Chan JK, Tang C, et al. Low-grade B-cell lymphoma of mucosa-associated lymphoid tissue arising in the thymus. A thymic lymphoma mimicking myoepithelial sialadenitis. Am J Surg Pathol 1990;14:342-51. [Crossref] [PubMed]
- Inagaki H, Chan JK, Ng JW, et al. Primary thymic extranodal marginal-zone B-cell lymphoma of mucosa-associated lymphoid tissue type exhibits distinctive clinicopathological and molecular features. Am J Pathol 2002;160:1435-43. [Crossref] [PubMed]
- Sunohara M, Hara K, Osamura K, et al. Mucosa associated lymphoid tissue (MALT) lymphoma of the thymus with trisomy 18. Intern Med 2009;48:2025-32. [Crossref] [PubMed]
- Piña-Oviedo S, Moran CA. Primary Mediastinal Classical Hodgkin Lymphoma. Adv Anat Pathol 2016;23:285-309. [Crossref] [PubMed]
- Rosenwald A, Wright G, Leroy K, et al. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med 2003;198:851-62. [Crossref] [PubMed]
- Harris NL. Hodgkin's disease: classification and differential diagnosis. Mod Pathol 1999;12:159-75. [PubMed]
- Savage KJ, Monti S, Kutok JL, et al. The molecular signature of mediastinal large B-cell lymphoma differs from that of other diffuse large B-cell lymphomas and shares features with classical Hodgkin lymphoma. Blood 2003;102:3871-9. [Crossref] [PubMed]
- Hoeller S, Zihler D, Zlobec I, et al. BOB.1, CD79a and cyclin E are the most appropriate markers to discriminate classical Hodgkin's lymphoma from primary mediastinal large B-cell lymphoma. Histopathology 2010;56:217-28. [Crossref] [PubMed]
- Sarkozy C, Molina T, Ghesquières H, et al. Mediastinal gray zone lymphoma: clinico-pathological characteristics and outcomes of 99 patients from the Lymphoma Study Association. Haematologica 2017;102:150-9. [Crossref] [PubMed]
- O'Malley DP, Fedoriw Y, Weiss LM. Distinguishing Classical Hodgkin Lymphoma, Gray Zone Lymphoma, and Large B-cell Lymphoma: A Proposed Scoring System. Appl Immunohistochem Mol Morphol 2016;24:535-40. [Crossref] [PubMed]
- Hutchinson CB, Wang E. Primary mediastinal (thymic) large B-cell lymphoma: a short review with brief discussion of mediastinal gray zone lymphoma. Arch Pathol Lab Med 2011;135:394-8. [PubMed]
- Traverse-Glehen A, Pittaluga S, Gaulard P, et al. Mediastinal gray zone lymphoma: the missing link between classic Hodgkin's lymphoma and mediastinal large B-cell lymphoma. Am J Surg Pathol 2005;29:1411-21. [Crossref] [PubMed]
- Menestrina F, Chilosi M, Bonetti F, et al. Mediastinal large-cell lymphoma of B-type, with sclerosis: histopathological and immunohistochemical study of eight cases. Histopathology 1986;10:589-600. [Crossref] [PubMed]
- Copie-Bergman C, Gaulard P, Maouche-Chrétien L, et al. The MAL gene is expressed in primary mediastinal large B-cell lymphoma. Blood 1999;94:3567-75. [PubMed]
- Copie-Bergman C, Plonquet A, Alonso MA, et al. MAL expression in lymphoid cells: further evidence for MAL as a distinct molecular marker of primary mediastinal large B-cell lymphomas. Mod Pathol 2002;15:1172-80. [Crossref] [PubMed]
- Joos S, Otaño-Joos MI, Ziegler S, et al. Primary mediastinal (thymic) B-cell lymphoma is characterized by gains of chromosomal material including 9p and amplification of the REL gene. Blood 1996;87:1571-8. [PubMed]
- Bentz M, Barth TF, Brüderlein S, et al. Gain of chromosome arm 9p is characteristic of primary mediastinal B-cell lymphoma (MBL): comprehensive molecular cytogenetic analysis and presentation of a novel MBL cell line. Genes Chromosomes Cancer 2001;30:393-401. [Crossref] [PubMed]
- Eberle FC, Salaverria I, Steidl C, et al. Gray zone lymphoma: chromosomal aberrations with immunophenotypic and clinical correlations. Mod Pathol 2011;24:1586-97. [Crossref] [PubMed]
- Steidl C, Gascoyne RD. The molecular pathogenesis of primary mediastinal large B-cell lymphoma. Blood 2011;118:2659-69. [Crossref] [PubMed]
- Eberle FC, Rodriguez-Canales J, Wei L, et al. Methylation profiling of mediastinal gray zone lymphoma reveals a distinctive signature with elements shared by classical Hodgkin's lymphoma and primary mediastinal large B-cell lymphoma. Haematologica 2011;96:558-66. [Crossref] [PubMed]
- Kritharis A, Pilichowska M, Evens AM. How I manage patients with grey zone lymphoma. Br J Haematol 2016;174:345-50. [Crossref] [PubMed]
- Pilichowska M, Pittaluga S, Ferry JA, et al. Clinicopathologic consensus study of gray zone lymphoma with features intermediate between DLBCL and classical HL. Blood Adv 2017;1:2600-9. [Crossref] [PubMed]
- McCluggage WG, McManus K, Qureshi R, et al. Low-grade B-cell lymphoma of mucosa-associated lymphoid tissue (MALT) of thymus. Hum Pathol 2000;31:255-9. [Crossref] [PubMed]
- Kim JM. Primary extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue-type in the thymus of a patient with Sjögren's syndrome and rheumatoid arthritis. J Korean Med Sci 2003;18:897-900. [Crossref] [PubMed]
- Go H, Cho HJ, Paik JH, et al. Thymic extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue: a clinicopathological and genetic analysis of six cases. Leuk Lymphoma 2011;52:2276-83. [Crossref] [PubMed]
- Kang LY, Ho SP, Chou YP. Primary thymic mucosa-associated lymphoid tissue lymphoma with multiple thin walled lung cysts: case report and literature review. Chin J Cancer Res 2013;25:354-7. [PubMed]
- Arai H, Tajiri M, Kaneko S, et al. Two surgical cases of thymic MALT lymphoma associated with multiple lung cysts: possible association with Sjögren's syndrome. Gen Thorac Cardiovasc Surg 2017;65:229-34. [Crossref] [PubMed]
- Isaacson PG. Gastrointestinal lymphoma. Hum Pathol 1994;25:1020-9. [Crossref] [PubMed]
- Wakely P, Suster S. Langerhans' cell histiocytosis of the thymus associated with multilocular thymic cyst. Hum Pathol 2000;31:1532-5. [Crossref] [PubMed]
- Chan JK, Fletcher CD, Nayler SJ, et al. Follicular dendritic cell sarcoma. Clinicopathologic analysis of 17 cases suggesting a malignant potential higher than currently recognized. Cancer 1997;79:294-313. [Crossref] [PubMed]
- Lin O, Frizzera G. Angiomyoid and follicular dendritic cell proliferative lesions in Castleman's disease of hyaline-vascular type: a study of 10 cases. Am J Surg Pathol 1997;21:1295-306. [Crossref] [PubMed]
- Desai SB, Pradhan SA, Chinoy RF. Mediastinal Castleman's disease complicated by follicular dendritic cell tumour. Indian J Cancer 2000;37:129-32. [PubMed]
- Castleman B, Iverson L, Menendez VP. Localized mediastinal lymphnode hyperplasia resembling thymoma. Cancer 1956;9:822-30. [Crossref] [PubMed]
- Frizzera G, Banks PM, Massarelli G, et al. A systemic lymphoproliferative disorder with morphologic features of Castleman's disease. Pathological findings in 15 patients. Am J Surg Pathol 1983;7:211-31. [Crossref] [PubMed]
- Weisenburger DD, Nathwani BN, Winberg CD, et al. Multicentric angiofollicular lymph node hyperplasia: a clinicopathologic study of 16 cases. Hum Pathol 1985;16:162-72. [Crossref] [PubMed]
- Dispenzieri A, Armitage JO, Loe MJ, et al. The clinical spectrum of Castleman's disease. Am J Hematol 2012;87:997-1002. [Crossref] [PubMed]
- Carbone A, De Paoli P, Gloghini A, et al. KSHV-associated multicentric Castleman disease: A tangle of different entities requiring multitarget treatment strategies. Int J Cancer 2015;137:251-61. [Crossref] [PubMed]
- Castleman B. Tumors of the thymus gland First series ed. Atlas of Tumor pathology, vol 19. Washington, DC: Armed Forces Institute of Pathology, 1955.
- Middleton G. Involvement of the thymus by metastic neoplasms. Br J Cancer 1966;20:41-6. [Crossref] [PubMed]
- Suster S, Moran C. Diagnostic pathology: Thoracic. 1st ed. Diagnostic pathology. Canada: Amirsys Publishing, Inc., 2012.
- Ahuja S, Ernst H, Lenz K. Papillary thyroid carcinoma: occurrence and types of lymph node metastases. J Endocrinol Invest 1991;14:543-9. [Crossref] [PubMed]
- Dralle H, Damm I, Scheumann GF, et al. Frequency and significance of cervicomediastinal lymph node metastases in medullary thyroid carcinoma: results of a compartment-oriented microdissection method. Henry Ford Hosp Med J 1992;40:264-7. [PubMed]
- Girard N, Ruffini E, Marx A, et al. Thymic epithelial tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26:v40-55. [Crossref] [PubMed]
- Moran CA, Suster S. On the histologic heterogeneity of thymic epithelial neoplasms. Impact of sampling in subtyping and classification of thymomas. Am J Clin Pathol 2000;114:760-6. [Crossref] [PubMed]
- Pan CC, Chen PC, Chou TY, et al. Expression of calretinin and other mesothelioma-related markers in thymic carcinoma and thymoma. Hum Pathol 2003;34:1155-62. [Crossref] [PubMed]
- Pomplun S, Wotherspoon AC, Shah G, et al. Immunohistochemical markers in the differentiation of thymic and pulmonary neoplasms. Histopathology 2002;40:152-8. [Crossref] [PubMed]
- Attanoos RL, Galateau-Salle F, Gibbs AR, et al. Primary thymic epithelial tumours of the pleura mimicking malignant mesothelioma. Histopathology 2002;41:42-9. [Crossref] [PubMed]
- Nonaka D, Henley JD, Chiriboga L, et al. Diagnostic utility of thymic epithelial markers CD205 (DEC205) and Foxn1 in thymic epithelial neoplasms. Am J Surg Pathol 2007;31:1038-44. [Crossref] [PubMed]
- Kriegsmann M, Muley T, Harms A, et al. Differential diagnostic value of CD5 and CD117 expression in thoracic tumors: a large scale study of 1465 non-small cell lung cancer cases. Diagn Pathol 2015;10:210. [Crossref] [PubMed]
- Kim BS, Kim JK, Kang CH, et al. An immunohistochemical panel consisting of EZH2, C-KIT, and CD205 is useful for distinguishing thymic squamous cell carcinoma from type B3 thymoma. Pathol Res Pract 2018;214:343-9. [Crossref] [PubMed]
- Wakely PE. Fine needle aspiration in the diagnosis of thymic epithelial neoplasms. Hematol Oncol Clin North Am 2008;22:433-42. [Crossref] [PubMed]
- Dixit R, Shah NS, Goyal M, et al. Diagnostic evaluation of mediastinal lesions: Analysis of 144 cases. Lung India 2017;34:341-8. [Crossref] [PubMed]
- Menter T, Gasser A, Juskevicius D, et al. Diagnostic Utility of the Germinal Center-associated Markers GCET1, HGAL, and LMO2 in Hematolymphoid Neoplasms. Appl Immunohistochem Mol Morphol 2015;23:491-8. [Crossref] [PubMed]
- Jevremovic D, Roden AC, Ketterling RP, et al. LMO2 Is a Specific Marker of T-Lymphoblastic Leukemia/Lymphoma. Am J Clin Pathol 2016;145:180-90. [Crossref] [PubMed]
- Jegalian AG, Bodo J, Hsi ED. NOTCH1 intracellular domain immunohistochemistry as a diagnostic tool to distinguish T-lymphoblastic lymphoma from thymoma. Am J Surg Pathol 2015;39:565-72. [Crossref] [PubMed]
- Ito J, Yoshida A, Maeshima AM, et al. Concurrent thymoma, thymic carcinoma, and T lymphoblastic leukemia/lymphoma in an anterior mediastinal mass. Pathol Res Pract 2015;211:693-6. [Crossref] [PubMed]
- Otton SH, Standen GR, Ormerod IE. T cell lymphocytosis associated with polymyositis, myasthenia gravis and thymoma. Clin Lab Haematol 2000;22:307-8. [Crossref] [PubMed]
- Kon T, Mori F, Tanji K, et al. Giant cell polymyositis and myocarditis associated with myasthenia gravis and thymoma. Neuropathology 2013;33:281-7. [Crossref] [PubMed]
- Kakuta N, Sumitani M, Sugitani A, et al. Mediastinal peripheral T-cell lymphoma diagnosed by repeated biopsies after an initial diagnosis of fibrosing mediastinitis. Respirol Case Rep 2017;5:e00272 [Crossref] [PubMed]
- Sander CA, Jaffe ES, Gebhardt FC, et al. Mediastinal lymphoblastic lymphoma with an immature B-cell immunophenotype. Am J Surg Pathol 1992;16:300-5. [Crossref] [PubMed]
- Maitra A, McKenna RW, Weinberg AG, et al. Precursor B-cell lymphoblastic lymphoma. A study of nine cases lacking blood and bone marrow involvement and review of the literature. Am J Clin Pathol 2001;115:868-75. [Crossref] [PubMed]
- Weissferdt A, Moran CA. Thymic hyperplasia with lymphoepithelial sialadenitis (LESA)-like features: a clinicopathologic and immunohistochemical study of 4 cases. Am J Clin Pathol 2012;138:816-22. [Crossref] [PubMed]
- Arndt B, Gaiser T, Marx A, et al. Lymphoepithelial sialadenitis (LESA)-like Thymic hyperplasia: A case report. Dtsch Med Wochenschr 2016;141:1026-9. [PubMed]
- Marino M, Martini M, Casini B, et al. An unusual case of Thymic MALT-type lymphoma. Eur J Pathol 2015;467:S262.
- Ströbel P, Marino M, Feuchtenberger M, et al. Micronodular thymoma: an epithelial tumour with abnormal chemokine expression setting the stage for lymphoma development. J Pathol 2005;207:72-82. [Crossref] [PubMed]
- Makis W, Hickeson M, Derbekyan V. Myeloid sarcoma presenting as an anterior mediastinal mass invading the pericardium: Serial Imaging With F-18 FDG PET/CT. Clin Nucl Med 2010;35:706-9. [Crossref] [PubMed]
- Lee JM, Song HN, Kang Y, et al. Isolated mediastinal myeloid sarcoma successfully treated with chemoradiotherapy followed by unrelated allogeneic stem cell transplantation. Intern Med 2011;50:3003-7. [Crossref] [PubMed]
- Wong WS, Loong F, Ooi GC, et al. Primary granulocytic sarcoma of the mediastinum. Leuk Lymphoma 2004;45:1931-3. [Crossref] [PubMed]
- Chevallier P, Labopin M, Cornelissen J, et al. Allogeneic hematopoietic stem cell transplantation for isolated and leukemic myeloid sarcoma in adults: a report from the Acute Leukemia Working Party of the European group for Blood and Marrow Transplantation. Haematologica 2011;96:1391-4. [Crossref] [PubMed]
- Pileri SA, Ascani S, Cox MC, et al. Myeloid sarcoma: clinico-pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia 2007;21:340-50. [Crossref] [PubMed]
- Catinella FP, Boyd AD, Spencer FC. Intrathoracic extramedullary hematopoiesis simulating anterior mediastinal tumor. J Thorac Cardiovasc Surg 1985;89:580-4. [PubMed]
- Santini M, Fiorelli A, Vicidomini G, et al. Intrathoracic extramedullary haematopoiesis manifested as a neoplastic lesion within anterior mediastinum. Ann Thorac Surg 2009;88:2001-4. [Crossref] [PubMed]
- Zhao GQ, Dowell JE. Hematologic malignancies associated with germ cell tumors. Expert Rev Hematol 2012;5:427-37. [Crossref] [PubMed]
- Bremmer F, Ströbel P. Pathologe 2016;37:441-8. [Mediastinal germ cell tumors]. [Crossref] [PubMed]
- Salah S, Al-Ibraheem A, Daboor A, et al. Synovial sarcoma presenting with huge mediastinal mass: a case report and review of literature. BMC Res Notes 2013;6:240. [Crossref] [PubMed]
- Salah S, Salem A. Primary synovial sarcomas of the mediastinum: a systematic review and pooled analysis of the published literature. ISRN Oncol 2014;2014:412527 [Crossref] [PubMed]
- Tsubochi H, Endo T, Sogabe M, et al. Solitary fibrous tumor of the thymus with variegated epithelial components. Int J Clin Exp Pathol 2014;7:7477-84. [PubMed]
- Zhou H, Zhong C, Fu Q, et al. Thoracoscopic resection of a huge mediastinal cystic lymphangioma. J Thorac Dis 2017;9:E887-9. [Crossref] [PubMed]
- Licci S, Puma F, Sbaraglia M, et al. Primary intrathymic lymphangioma. Am J Clin Pathol 2014;142:683-8. [Crossref] [PubMed]
- Zhang S, Zheng Y, Liu W, et al. Primary epithelioid angiosarcoma of the pleura: a case report and review of literature. Int J Clin Exp Pathol 2015;8:2153-8. [PubMed]
- Detlefsen S. IgG4-related disease: a systemic condition with characteristic microscopic features. Histol Histopathol 2013;28:565-84. [PubMed]
- Marino M, Lattanzio R, Pescarmona E. Mediastinal tumor markers. In: LoCicero J, Feins R, Rocco G, et al. editors. Shield's General Thoracic Surgery. Wolters Kluwer Health, 2018.
- Ishimoto H, Yatera K, Shimabukuro I, et al. Case of immunoglobulin G4 (IgG4)-related disease diagnosed by transbronchial lung biopsy and endobronchial ultrasound-guided transbronchial needle aspiration. J UOEH 2014;36:237-42. [Crossref] [PubMed]
- Taki M, Inada S, Ariyasu R, et al. Anaplastic large cell lymphoma mimicking fibrosing mediastinitis. Intern Med 2013;52:2645-51. [Crossref] [PubMed]
- Cingam SR, Al Shaarani M, Takalkar A, et al. Follicular dendritic sarcoma masquerading as fibrosing mediastinitis. BMJ Case Rep 2017;2017.
- Crotty TB, Colby TV, Gay PC, et al. Desmoplastic malignant mesothelioma masquerading as sclerosing mediastinitis: a diagnostic dilemma. Hum Pathol 1992;23:79-82. [Crossref] [PubMed]
- Parish JM, Rosenow EC. Mediastinal granuloma and mediastinal fibrosis. Semin Respir Crit Care Med 2002;23:135-43. [Crossref] [PubMed]
- Gorospe L, Ayala-Carbonero AM, Fernández-Méndez M, et al. Idiopathic fibrosing mediastinitis: spectrum of imaging findings with emphasis on its association with IgG4-related disease. Clin Imaging 2015;39:993-9. [Crossref] [PubMed]
- Rossi GM, Emmi G, Corradi D, et al. Idiopathic Mediastinal Fibrosis: a Systemic Immune-Mediated Disorder. A Case Series and a Review of the Literature. Clin Rev Allergy Immunol 2017;52:446-59. [Crossref] [PubMed]
- Weissferdt A, Hernandez JC, Kalhor N, et al. Spindle cell thymomas: an immunohistochemical study of 30 cases. Appl Immunohistochem Mol Morphol 2011;19:329-35. [Crossref] [PubMed]
- Lan T, Chen H, Xiong B, et al. Primary pleuropulmonary and mediastinal synovial sarcoma: a clinicopathologic and molecular study of 26 genetically confirmed cases in the largest institution of southwest China. Diagn Pathol 2016;11:62. [Crossref] [PubMed]
- Terry J, Saito T, Subramanian S, et al. TLE1 as a diagnostic immunohistochemical marker for synovial sarcoma emerging from gene expression profiling studies. Am J Surg Pathol 2007;31:240-6. [Crossref] [PubMed]
- Jagdis A, Rubin BP, Tubbs RR, et al. Prospective evaluation of TLE1 as a diagnostic immunohistochemical marker in synovial sarcoma. Am J Surg Pathol 2009;33:1743-51. [Crossref] [PubMed]
- Tajima S, Takahashi T, Itaya T, et al. Cystic synovial sarcoma of the pleura mimicking a cystic thymoma: a case report illustrating the role of decreased INI-1 expression in differential diagnosis. Int J Clin Exp Pathol 2015;8:3262-9. [PubMed]
- Nonaka D, Rosai J. Is there a spectrum of cytologic atypia in type a thymomas analogous to that seen in type B thymomas? A pilot study of 13 cases. Am J Surg Pathol 2012;36:889-94. [Crossref] [PubMed]
- Vladislav IT, Gökmen-Polar Y, Kesler KA, et al. The role of histology in predicting recurrence of type A thymomas: a clinicopathologic correlation of 23 cases. Mod Pathol 2013;26:1059-64. [Crossref] [PubMed]
Cite this article as: Marino M, Ascani S. An overview on the differential diagnostics of tumors of the anterior-superior mediastinum: the pathologist’s perspective. Mediastinum 2019;3:6.


