
Everolimus in thymic epithelial tumors: practical considerations
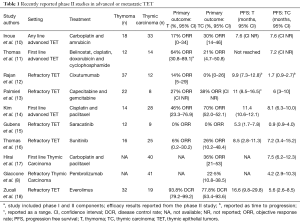
Thymoma and thymic carcinoma are known as thymic epithelial tumors (TETs) given their shared origin as epithelial neoplasms arising from the thymus. The understanding of the relationship between thymoma and thymic carcinoma has evolved over time. Thymic carcinoma was initially classified as type C thymoma in the 1999 World Health Organization (WHO) classification (1), but was separated as its own entity in later WHO classifications (2). Although thymoma is the most common tumor of the anterior mediastinum, it is rare with a reported incidence of 0.15 per 100,000 person-years (3). Thymic carcinoma is rarer still, accounting for perhaps 10% of all TETs. Surgery is the only curative intervention for both thymoma and thymic carcinoma, with chemoradiation offered to patients with unresectable disease (4-6). Palliative platinum-based chemotherapy is offered for patients with metastatic disease, however there are no standard treatment options for patients who progress on chemotherapy (7,8). The rarity of thymic carcinoma in particular has posed a major barrier to clinical trial development and accrual, resulting in a lack of robust clinical evidence to guide treatment decisions. One of the largest series in thymic carcinoma patients at a high volume center included 135 patients over 30 years, and confirmed the aggressive nature of thymic carcinoma, which is more often diagnosed at a later stage and has a poorer prognosis than the more indolent thymoma (9). Recently, several phase II studies have been reported in TET with some agents demonstrating promising efficacy (Table 1).
Full table
In nearly all of these studies, TET are combined for the purpose of efficacy analysis. Only two recent studies were limited to patients with thymic carcinoma, the largest of which accrued 41 patients. Given the unique challenges of studying this clinical entity, Zucali and collaborators are to be congratulated on accruing a total of 51 patients with TET over 2 years to their phase II study of everolimus in patients with refractory TET, including 19 patients with thymic carcinoma, resulting in one of the largest prospective clinical studies of patients with TET to date (18). This single arm, multicenter study revealed a high disease control rate (DCR) for patients with TET treated with everolimus, but unique toxicities occurred including fatal pneumonitis.
Everolimus is an oral MTOR-inhibitor that is approved for use in renal cell carcinoma, neuroendocrine tumors, and breast cancer, among others (19,20). Despite response rates typically under 5%, treatment with everolimus has resulted in prolonged periods of stable disease for patients with these cancers. Preclinical data suggesting a role of PI3K in TET, as well as promising reports of clinical activity in this patient population, led Zucali and collaborators to evaluate the role of everolimus in the treatment of patients with refractory TET (21,22). This phase II study included 51 patients with TET, 50 of whom received study drug (32 patients with thymoma, 18 patients with thymic carcinoma). This was a single-arm, open-label, multi-center phase II study of everolimus dosed at 10 mg daily continuously in patients with TET who had progressed on prior platinum-based chemotherapy. The primary objective was to evaluate the efficacy of everolimus in this patient population, with a primary endpoint of DCR including complete and partial responses and stable disease. Secondary endpoints included progression free survival (PFS), overall survival (OS), duration of response, and safety, as well as time to treatment failure (TTF), although TTF was not a pre-planned secondary endpoint. Overall, 50 patients were included in the intention-to-treat analysis, however only 46 patients were evaluable for response to treatment after 4 patients discontinued therapy prior to imaging due to either drug-related adverse events (n=3) or non-drug-related events (n=1). Disease control was observed in 44 of 50 patients for a DCR of 88% [95% confidence interval (CI), 78.7–95.5%]. Five partial responses were observed (10%) and one complete response was seen (2%) in a patient with thymic carcinoma. The median duration of response was 7.1 months (range, 1.2–25.9 months). Disease control rate varied by histology, observed in 30 patients with thymoma (93.8%; 95% CI, 79.2–99.2%) compared with 14 patients with thymic carcinoma (77.8%; 95% CI, 52.4–93.6%). For the 6 patients who experienced either a PR or CR, the durability of response differed for patients with thymoma (3.3, 25.5, 29.9 months) compared to those patients with thymic carcinoma (1.2, 5.9, 8.3 months). The time to treatment failure overall was 8.4 months (0.66–44.3 months) and also varied for patients with thymoma (median 11.3 months) compared to thymic carcinoma (median 5.6 months, P=0.001). The median OS overall was 25.7 months for the whole population (95% CI, 16–NR); median OS was not reached for thymoma patients and was 14.7 months (95% CI, 3.5–24 months) for patients with thymic carcinoma. In correlative studies, tumor positivity for p4E-BP1 and IGF1-R were associated with poorer survival, and expression of p4E-BP1 was higher in thymic carcinoma patients than in thymoma patients (57% vs. 10%, P=0.003). However, the study did not identify any predictive biomarkers for response to everolimus.
The most common toxicities reported were stomatitis (n=33, 66%), fatigue (n=24, 48%), mucositis (n=18, 36%) and pneumonitis (n=18, 36%), and the rate of serious toxicities (grade 3 or 4) was 28% (n=14). Overall, 70% of patients required dose interruption, 18% required permanent discontinuation of treatment due to toxicity, and 28% required dose reduction. The high rate of pneumonitis in the study is certainly concerning. Most worrying was the occurrence of fatal pneumonitis in 3 patients (6%). There were no clear risk factors to identify patients at risk for pneumonitis, although it occurred more commonly in thymoma than thymic carcinoma (P=0.064).
The results of this study must be put into the context of the changing landscape of treatment options for patients with refractory TET. As can be seen in Table 1, there are several promising new agents available for this patient population, including both targeted agents and immunotherapies. Given the high expression of PD-L1 in TET (23-25), checkpoint inhibitor immunotherapy has emerged as a possible treatment approach in patients with TET (8), but the risk of immune-related adverse events including pneumonitis and myocarditis must be weighed against any potential benefit of these therapies. With multiple new agents being evaluated, the optimal sequencing of these therapies has yet to be determined. The high rate of pneumonitis in patients treated with everolimus should be considered in the sequencing of treatment options, since the risk of pneumonitis or other immune-related toxicities with subsequent immunotherapy is unknown. In the current study, no patients were reported to have received treatment with prior immunotherapy. Additionally, unique immune toxicities have occurred in patients with TET, including a higher risk of myocarditis than in other patient populations (8), which may be affected by prior or subsequent therapies.
In a separate study, Thomas and collaborators conducted a phase II study of sunitinib in TET and reported an objective response rate (ORR) of 26% in 25 patients with thymic carcinoma. DCR was 91% (95% CI, 72.0–98.9%) in patients with thymic carcinoma and 81% (95% CI, 54.4–96.0%) in patients with thymoma. Median PFS was 7.2 months for patients with thymic carcinoma treated with sunitinib (Table 1), and after a median follow-up of 17 months, median OS was not reached for patients with thymic carcinoma and 15.5 months for patients with thymoma (95% CI, 12.6–NR). Grade 3 or 4 adverse events occurred in 70% of patients treated with sunitinib (n=28), including fatigue and mucositis in 8 each (20%), and 3 patients (8%) died during treatment with sunitinib due to progressive disease, sepsis, and treatment-related ventricular fibrillation. Importantly, the median duration of response for patients with thymic carcinoma was 16.4 months (range, 1.4–16.4 months), which is considerably longer than that experienced by patients treated with everolimus, acknowledging the limitations of cross-trial comparisons. Both everolimus and sunitinib were associated with high rates of serious toxicities, with pneumonitis being more common in patients treated with everolimus and cardiac and skin toxicities being frequent in patients treated with sunitinib.
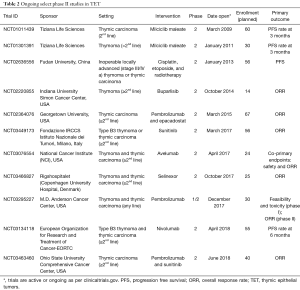
The vastly different outcomes in terms of response and survival between thymoma and thymic carcinoma in this study and others, as well as possible differences in toxicity to both targeted agents and immunotherapy, point to the importance of evaluating these entities separately. For instance, the recently reported phase II study of pembrolizumab accrued only patients with thymic carcinoma out of concern for exacerbating or precipitating paraneoplastic events in thymoma patients (8). Several ongoing phase II studies focus only on thymic carcinoma patients (Table 2). The rarity of these diseases should not preclude the ability to study them independently, which can be accomplished by leveraging multi-institutional collaborations in order to accrue patients, such as with the French RHYTMIC network or the International Thymic Malignancy Interest Group. In this context, Zucali et al. have demonstrated that accrual for a rare disease can still be accomplished in an acceptable time frame when done in collaboration.
Full table
In summary, Zucali and collaborators have demonstrated a high rate of disease control with everolimus in TET, including one complete response in a patient with thymic carcinoma. However, this came at the cost of a higher than expected rate of pneumonitis, including fatal pneumonitis in 3 (6%) of patients. The disparate outcomes by histology argue for the importance of including pre-planned efficacy analyses by histology, or better yet, designing studies for each histology independently. With several new treatment options being investigated for patients with thymoma and thymic carcinoma, the optimal sequencing of therapies is unknown and will be important to investigate in future studies.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by Section Editor Dr. Ningning Kang (Department of Thoracic Surgery, 1st Affiliated Hospital of Anhui Medical University, Hefei, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med.2018.07.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rosai J. Histological Typing of Tumours of the Thymus. Berlin: Springer, 1999.
- Marx A, Ströbel P, Badve SS, et al. ITMIG Consensus Statement on the Use of the WHO Histological Classification of Thymoma and Thymic Carcinoma: Refined Definitions, Histological Criteria, and Reporting. J Thorac Oncol 2014;9:596-611. [Crossref] [PubMed]
- Engels EA, Pfeiffer RM. Malignant thymoma in the United States: demographic patterns in incidence and associations with subsequent malignancies. Int J Cancer 2003;105:546-51. [Crossref] [PubMed]
- Girard N, Ruffini E, Marx A, et al. Thymic epithelial tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26:v40-55. [Crossref] [PubMed]
- Kim ES, Putnam JB, Komaki R, et al. Phase II study of a multidisciplinary approach with induction chemotherapy, followed by surgical resection, radiation therapy, and consolidation chemotherapy for unresectable malignant thymomas: final report. Lung Cancer 2004;44:369-79. [Crossref] [PubMed]
- Zhao Y, Shi J, Fan L, et al. Surgical treatment of thymoma: an 11-year experience with 761 patients. Eur J Cardiothorac Surg 2016;49:1144-9. [Crossref] [PubMed]
- Furugen M, Sekine I, Tsuta K, et al. Combination chemotherapy with carboplatin and paclitaxel for advanced thymic cancer. Jpn J Clin Oncol 2011;41:1013-6. [Crossref] [PubMed]
- Giaccone G, Kim C, Thompson J, et al. Pembrolizumab in patients with thymic carcinoma: a single-arm, single-centre, phase 2 study. Lancet Oncol 2018;19:347-55. [Crossref] [PubMed]
- Zhai Y, Hui Z, Ji W, et al. A Single-Center Analysis of the Treatment and Prognosis of Patients With Thymic Carcinoma. Ann Thorac Surg 2017;104:1718-24. [Crossref] [PubMed]
- Inoue A, Sugawara S, Harada M, et al. Phase II study of Amrubicin combined with carboplatin for thymic carcinoma and invasive thymoma: North Japan Lung Cancer group study 0803. J Thorac Oncol 2014;9:1805-9. [Crossref] [PubMed]
- Thomas A, Rajan A, Szabo E, et al. A phase I/II trial of belinostat in combination with cisplatin, doxorubicin, and cyclophosphamide in thymic epithelial tumors: a clinical and translational study. Clin Cancer Res 2014;20:5392-402. [Crossref] [PubMed]
- Rajan A, Carter CA, Berman A, et al. Cixutumumab for patients with recurrent or refractory advanced thymic epithelial tumours: a multicentre, open-label, phase 2 trial. Lancet Oncol 2014;15:191-200. [Crossref] [PubMed]
- Palmieri G, Buonerba C, Ottaviano M, et al. Capecitabine plus gemcitabine in thymic epithelial tumors: final analysis of a Phase II trial. Future Oncol 2014;10:2141-7. [Crossref] [PubMed]
- Kim HS, Lee JY, Lim SH, et al. A Prospective Phase II Study of Cisplatin and Cremophor EL-Free Paclitaxel (Genexol-PM) in Patients with Unresectable Thymic Epithelial Tumors. J Thorac Oncol 2015;10:1800-6. [Crossref] [PubMed]
- Gubens MA, Burns M, Perkins SM, et al. A phase II study of saracatinib (AZD0530), a Src inhibitor, administered orally daily to patients with advanced thymic malignancies. Lung Cancer 2015;89:57-60. [Crossref] [PubMed]
- Thomas A, Rajan A, Berman A, et al. Sunitinib in patients with chemotherapy-refractory thymoma and thymic carcinoma: an open-label phase 2 trial. Lancet Oncol 2015;16:177-86. [Crossref] [PubMed]
- Hirai F, Yamanaka T, Taguchi K, et al. A multicenter phase II study of carboplatin and paclitaxel for advanced thymic carcinoma: WJOG4207L. Ann Oncol 2015;26:363-8. [Crossref] [PubMed]
- Zucali PA, De Pas T, Palmieri G, et al. Phase II Study of Everolimus in Patients With Thymoma and Thymic Carcinoma Previously Treated With Cisplatin-Based Chemotherapy. J Clin Oncol 2018;36:342-9. [Crossref] [PubMed]
- Kornblum N, Zhao F, Manola J, et al. Randomized Phase II Trial of Fulvestrant Plus Everolimus or Placebo in Postmenopausal Women With Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer Resistant to Aromatase Inhibitor Therapy: Results of PrE0102. J Clin Oncol 2018;36:1556-63. [Crossref] [PubMed]
- Motzer RJ, Escudier B, Oudard S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer 2010;116:4256-65. [Crossref] [PubMed]
- Alberobello AT, Wang Y, Beerkens FJ, et al. PI3K as a Potential Therapeutic Target in Thymic Epithelial Tumors. J Thorac Oncol 2016;11:1345-56. [Crossref] [PubMed]
- Palmieri G, Buonerba C, Federico P, et al. Everolimus plus long-acting somatostatin analogs in thymic epithelial malignancies. World J Clin Oncol 2012;3:111-5. [Crossref] [PubMed]
- Katsuya Y, Fujita Y, Horinouchi H, et al. Immunohistochemical status of PD-L1 in thymoma and thymic carcinoma. Lung Cancer 2015;88:154-9. [Crossref] [PubMed]
- Owen D, Chu B, Lehman AM, et al. Expression patterns, prognostic value, and intratumoral heterogeneity of PD-L1 and PD-1 in thymoma and thymic carcinoma. J Thorac Oncol 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Yokoyama S, Miyoshi H, Nakashima K, et al. Prognostic Value of Programmed Death Ligand 1 and Programmed Death 1 Expression in Thymic Carcinoma. Clin Cancer Res 2016;22:4727-34. [Crossref] [PubMed]
Cite this article as: Owen DH, Otterson GA. Everolimus in thymic epithelial tumors: practical considerations. Mediastinum 2018;2:46.


