
The pathology of mesenchymal tumors of the mediastinum
Introduction and diagnostic approach to mediastinal neoplasms
Mesenchymal soft tissue tumors (MST) account for only 2% of all tumours in the mediastinum. With very few exceptions, their morphological and molecular features are identical to their respective counterparts elsewhere in the body, although the prognosis of MST seems to be more guarded due to therapeutic restrictions related to the complicated anatomy. Since the available literature on the epidemiological and molecular features and clinical presentation of MST have been extensively reviewed (1,2), we will focus here on the practical approach to the diagnosis of the most frequent MST and on the interpretation of growth patterns rather than on clinical or molecular issues. It cannot be overemphasized that consideration of clinical, epidemiological and anatomic background information is essential to reach a correct diagnosis. Many MST (as well as non-mesenchymal lesions that come into the differential diagnosis) occur in quite distinct anatomic locations (see Table 1), in typical age groups or with gender predilection, or have clinical clues (such as Myasthenia gravis or Neurofibromatosis type 1). However, in the era of personalized medicine, many of the entities discussed here require both an extended panel of immunohistochemical antibodies and molecular tests for their diagnosis (Table 2).
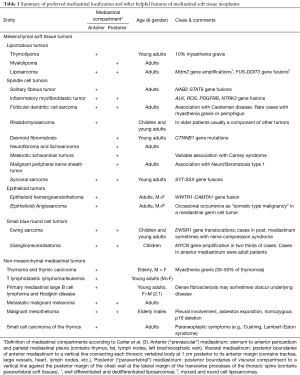
Full table

Full table
Neoplasms with a lipomatous component
Lipomatous tumors are frequent in the mediastinum and account for up to 10% of mediastinal masses. Especially in young adults with a mass lesion in the anterior mediastinum, thymolipoma is a major consideration. Presence of autoimmune phenomena (usually myasthenia gravis, but also autoimmune thyroiditis and others) is rare, but an important clue to the diagnosis. The diagnosis requires demonstration of thymic tissue in an adipose mass lesion, but the relative proportion of both components can be quite variable and absence of thymic tissue in a biopsy does not rule out this possibility. In elderly patients, thymic involution may be a differential diagnosis, but this condition does not form a mediastinal mass. Thymolipoma can go undetected for a long time and may be quite large (up to 30 cm), raising concerns of malignancy. Careful macroscopic grossing and histological sampling of all areas that differ in color or consistency is essential in such cases. In tumors of the posterior mediastinum, myelolipoma with a hematopoietic component may be a consideration (4).
Although the salient morphological features of well-differentiated liposarcoma (variable size of adipocytes, enlarged hyperchromatic nuclei, fibrous septa with a subtle inflammatory component) are absent in (thymo)lipoma, regressive changes can sometimes mimic these features. Moreover, spindle cell lipoma, a distinctive CD34+ lipoma variant with highly variable lipomatous differentiation, characterized by „ropey collagen“ (Figure 1A) and slender nuclei embedded in a sometimes myxoid stroma, can be quite cellular and may raise concern for malignancy (5,6). In these cases, immunohistochemistry for mdm2 and CDK4 or FISH probes for the detection of mdm2 gene high level amplifications on Chr. 12q13-21 are required. Well differentiated liposarcoma can show a sclerosing or inflammatory pattern with focal loss of lipocytic differentiation (Figure 1B), but cytological atypia is mild and mitotic counts are low. These variants must be distinguished from dedifferentiated liposarcoma (see below), which is characterized by a poorly differentiated sarcomatous morphology (Figure 1C,D).
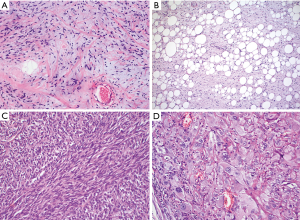
Ancillary studies are also usually required for the diagnosis of the two other liposarcoma types that may occur in the mediastinum, myxoid- and pleomorphic liposarcoma. Myxoid liposarcoma is characterized by a prominent myxoid stroma with relatively few spindle cells that may form a pseudo-alveolar pattern, and delicate capillaries with very characteristic branching. Importantly, the round cell variant of myxoid liposarcoma, a progression pattern of these tumors, has not been reported in the mediastinum. There is no specific immune phenotype and FISH analysis for the demonstration of DDIT3 rearrangements is required. Finally, pleomorphic liposarcoma, a high grade sarcoma with complex genetics and without mdm2 or DDIT3 abnormalities that usually occurs in older adults, has also been rarely described in the mediastinum (7). Pleomorphic liposarcoma is characterized by giant pleomorphic fat cells and may otherwise show overlapping features with dedifferentiated liposarcoma.
Neoplasms with spindle cell morphology
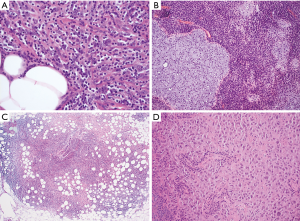
A majority of biopsies and resections specimens taken from the mediastinum will have a spindle cell morphology (Figure 2). The diagnosis should start with a review of the patient’s age and clinical presentation, as well as consideration of the anatomic location of the tumor. In a resection specimen, it is of crucial importance to carefully analyze adjacent tissues (blood vessels, bone, mediastinal fat and thymus etc.), since they can also offer valuable clues (for example, atypical fat cells may hint to a dedifferentiated liposarcoma). Next, the morphology and degree of cytologic atypia in the neoplastic cells and any inflammatory bystander cells should be determined. Careful characterization of the inflammatory infiltrate is important and may require immunohistochemistry: demonstration, e.g., of immature T-cells (preferably through TdT staining) in a spindle cell neoplasm more or less narrows the differential diagnosis down to thymoma and follicular dendritic cell (FDC) tumor/sarcoma (Figure 2A). Presence of a mixed infiltrate with eosinophils may hint to an inflammatory myofibroblastic tumor (IMFT). Of note, a sparse inflammatory infiltrate is also a consistent feature of well-differentiated liposarcoma (e.g., sclerotic or inflammatory variant), which is among the most frequent soft tissue sarcomas in virtually any organ and which should always be in the differential diagnosis. Although not the subject of this review, it should be noted that hematological neoplasms of the mediastinum can be either very sclerotic (primary mediastinal large B cell lymphoma) and/or may contain eosinophils (Hodgkin lymphoma, Langerhans cell histiocytosis), and should be considered in the differential diagnosis (Figure 2C).
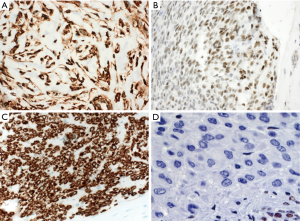
In a highly cellular tumor with compact spindle cells and mild to moderate atypia in the anterior mediastinum, the differential diagnosis should include type A or AB thymoma and synovial sarcoma. Synovial sarcoma tends to occur in young adults (median 35 years), while especially type A thymoma is usually a tumor of the elderly. Based on morphology alone, the distinction can be challenging if not impossible and requires immunohistochemistry and/or molecular tests (Figure 3). Both entities show expression of cytokeratins and even p63 (8), although strong diffuse expression of cytokeratins rather indicates a thymoma. Many thymomas with a spindle cell morphology (type A and AB) show focal ectopic expression of CD20, a feature not seen in synovial sarcoma. Many synovial sarcomas express CD56 and CD99 (9), and >90% express TLE1 (Figure 3B). Demonstration of a t(X;18) (SYT-SSX) rearrangement e.g. by FISH is currently the gold standard to render a definite diagnosis. Two other low grade spindle cell tumors with overlapping morphological features are solitary fibrous tumor (SFT) and IMFT (which can show sclerotic areas depleted of the characteristic inflammatory infiltrate). “Hemangiopericytoma-like” or “stag-horn” blood vessels are usually much more prominent in SFT, but can be found in both. A combination of CD34 and strong nuclear STAT6 expression is the hallmark of SFT (Figure 3A), although CD34 expression can be focal or absent. Smooth-muscle actin is usually not expressed in SFT and positive staining should trigger exclusion of IMFT especially in a young patient or a child. Although immunohistochemistry for ALK1 is a good screening marker for IMFT with ALK rearrangements, negative staining does not rule out IMFT, since only about 50% of cases are characterized by ALK rearrangements. Most of the ALK-negative tumors harbour chromosomal translocations that lead to “actionable” ROS1-WYHAE, PDGFRB-NAB2, and NTRK3-ETV6 gene fusions (10,11). Detection of these clinically relevant genetic alterations will usually require ancillary molecular studies such as FISH or NGS.
FDC sarcoma is an uncommon malignancy that may occur in the mediastinum or in mediastinal lymph nodes of adults and may be associated with paraneoplastic immune phenomena such as myasthenia gravis or pemphigus. Its morphology is variable, but quite characteristic with spindle cells in a fascicular or storiform growth pattern that may or may not show nuclear pleomorphism. A very characteristic and important finding is a mild to moderate lymphocytic component that often clusters around blood vessels (Figure 2A). These lymphocytes may show an immature phenotype with expression of TdT. A subset of cases is associated with hyaline-vascular Castleman disease which may coexist in the same tumor. The spindle cells are positive for FDC markers such as CD21, CD23, and CD35, while CD68 and S100 are variable and usually weakly expressed. D2-40 is positive in many cases. In contrast to thymoma, keratin is negative in FDC sarcoma.
The main differential diagnoses of spindle cell tumors with high grade morphology include malignant SFT, monophasic synovial sarcoma, dedifferentiated liposarcoma, (thymic) carcinoma, mesothelioma, melanoma and malignant melanotic schwannian tumors, and (preferentially in tumors of the posterior mediastinum) malignant peripheral nerve sheath tumor (MPNST). Especially in younger male patients, a sarcoma (usually rhabdomyosarcoma or angiosarcoma) arising as a so-called “somatic type malignancy” in a germ cell tumor may be a consideration. These secondary tumors have no distinctive histological features, but may contain small SALL4+ and/or OCT3/4+ remnants of the germ cell tumor. Since men with Klinefelter syndrome are at increased risk to develop mediastinal germ cell tumors, this is an important clinical information that should be asked for by the pathologist.
As discussed above, immunohistochemical demonstration of nuclear STAT6 expression is currently considered the most specific marker for SFT (Figure 3A), and nuclear TLE1 expression is a good screening marker for synovial sarcoma (Figure 3B), but should be confirmed by molecular tests (such as SYT FISH). Dedifferentiated liposarcoma is probably the most frequent malignant mesenchymal spindle cell tumor of the mediastinum and should always be in the differential diagnosis. Initial misdiagnoses are frequent due to its highly protean morphology, which can resemble fibromatosis and fibrosarcoma (Figure 1C), MPNST, tumors with epithelioid morphology such as melanoma, and undifferentiated pleomorphic sarcoma (type “MFH”) (Figure 1D). The diagnosis is further complicated by the fact that some cases can contain areas of heterologous differentiation such as rhabdomyosarcoma. Since a number of other tumors including MPNST, inflammatory myofibroblastic tumors (IMFTs), and germ cell tumors can show immunohistochemical overexpression of mdm2, only demonstration of mdm2 gene amplification by, e.g., by FISH can be considered specific for the diagnosis of dedifferentiated liposarcoma at this time. Malignant melanoma is another malignancy notorious for its highly variable histomorphology and lavish use of respective antibodies (such as S100, MelanA, and Sox10) is recommended. (Malignant) melanotic schwannoma is a distinctive neoplasm that usually arises in the spinal nerve roots and is thus located in the posterior mediastinum. Some cases (preferentially in young adults) are associated with Carney syndrome (myxomas of the heart and skin, epithelioid blue nevi, Cushing disease and acromegaly). The concept of melanotic schwannoma nicely illustrates the embryologic relationship between Schwann cells and melanocytes and is intermediate between a Schwann cell neoplasm and a melanoma with sometimes heavy pigmentation and expression of melanotic markers. Nuclear atypia, increased mitotic activity and absence of psammomatous calcifications are the main criteria used to differentiate benign melanotic schwannomas from malignant cases.
The diagnosis of malignant mesothelioma, especially of the sarcomatoid and desmoplastic variants, can be a major challenge and may sometimes be one of exclusion, since the overlap with other entities is huge and typical mesothelial markers are unreliable. Focal keratin expression, positivity for BAP1, detection of a homozygous p16 deletion e.g. by FISH, and close correlation with the clinical presentation (pleural thickening on imaging studies, history of asbestos exposition) are important clues.
Sarcomatoid (thymic) carcinoma (a poorly differentiated (thymic) carcinoma with spindle cell morphology) and carcinosarcoma (a tumor with both a malignant epithelial and a spindle cell/sarcomatous component) are also mainly defined by their variable expression of keratins and EMA, and of markers that indicate a thymic origin such as CD5 and CD117. The carcinomatous component may consist of squamous cell carcinoma, adenocarcinoma, or undifferentiated carcinoma. The sarcomatous component is usually spindle cell but may show heterologous differentiation (e.g., rhabdomyoblasts or osteoid). Many cases of sarcomatoid carcinomas contained a component of type A thymoma (12,13), which may be an important clue. Metaplastic thymoma is another biphasic tumor that lacks significant nuclear atypia in the spindle cell component and does not express CD5 and CD117.
MPNST typically arise in the posterior mediastinum of adult patients and have often contact to spinal nerve roots. About half of the cases are associated with neurofibromatosis type 1 (NF1). However, especially in small biopsies, a definitive diagnosis may not be possible due to lack of specific markers. The diagnosis is further complicated by the fact that approximately 15% of cases show heterologous (e.g., epithelial/glandular, chondroid, rhabdomyoid, vascular, etc.) differentiation (Figure 2B). The typical growth pattern corresponds to what has formerly been called fibrosarcoma: slender spindle cells in a fascicular arrangement, often with alternation between cellular and less cellular areas, creating a vaguely nodular or “marbleized” appearance. Protrusion or “herniation” of tumor into blood vessels is a characteristic finding that may however not be present in all cases. Another typical although non-specific pattern is extensive tumor necrosis with preservation of perivascular areas. Well differentiated areas may resemble other nerve sheath tumors such as schwannomas or neurofibromas. Remnants of a neurofibroma in the vicinity of the tumor as well as intra- or perineural growth are strong diagnostic clues. It must be emphasized that most MPNST (with the exception of the epithelioid variant and the so-called perineurial variant) show only focal and/or weak expression of classical Schwann cell markers such as S100 and Sox10 (epithelioid MPNST) (14), as well as reduced numbers of CD34-positive fibroblasts (perineurial MPNST). Thus, in a biopsy, a diagnosis of MPNST should not be made in a spindle cell neoplasm with strong expression of S100 because of potential confusion with benign nerve sheath tumors such as cellular schwannoma. Features that are not helpful in the diagnosis of MPNST per se, but in the distinction between a benign and a malignant nerve sheath tumor include loss of p16 expression, strong nuclear p53 staining and complete loss of H3K27me expression (14). Contrary to spindle cell MPNST, the epithelioid variant is generally diffusely and strongly positive for S100 and Sox10 and in addition about two-thirds of epithelioid MPNSTs will show loss of INI1 (Figure 3D).
Neoplasms with epithelioid morphology
Major differential diagnoses in this group include thymoma, lymphomas such as primary mediastinal large B cell lymphoma or anaplastic large cell lymphoma, melanoma, mesothelioma, epithelioid SFT, epithelioid MPNST, biphasic synovial sarcoma, and vascular tumors such as epithelioid hemangioendothelioma (EHE) and epithelioid angiosarcoma. Metastatic melanoma may have a striking epithelioid morphology and may sometimes resemble thymoma or thymic carcinoma. Since melanomas can express CD117 and even (focally) keratin (Figure 2D), this distinction is not always straightforward and requires inclusion of melanocytic markers such as MelanA and SOX10.
Epithelioid hemangioendotheliomas (EHE) are rare tumors arising in the anterior mediastinum of adults with a slight male preponderance and may have contact to large blood vessels (superior vena cava or innominate vein). Due to their strikingly epithelioid morphology and expression of keratin in about one third of cases (15), an initial misdiagnosis as metastatic carcinoma is very frequent. An important clue is presence of a chondroid or sometimes myxoid stroma and “blistering” of tumor cells with intracytoplasmic and intranuclear inclusions. EHE show strong expression of vascular markers such as CD31 and ERG (which are preferred to CD34 due to its frequent loss in vascular tumors and its expression in many other entities), and harbour a diagnostic WWTR1-CAMTA1 gene fusion [that can be detected by antibodies or by FISH (15)]. The distinction of EHE from epithelioid angiosarcoma on morphological grounds alone may be difficult if not impossible in small biopsies: features that favour angiosarcoma include high grade cytology, lack of myxoid/chondroid stroma, and slit like anastomosing blood vessels. CAMTA1 abnormalities are absent in angiosarcomas and thus offer a reliable means to distinguish both entities (15). As pointed out above, in a male patient, angiosarcoma may occur as a “somatic type malignancy” in a person with Klinefelter syndrome and a mediastinal germ cell tumor.
Pleomorphic and small cell neoplasms
The differential diagnosis of pleomorphic neoplasms comprises many of the entities discussed above, including poorly differentiated or undifferentiated thymic carcinoma, mesothelioma, melanoma, or myeloid sarcoma (chloroma).
Cases of giant cell tumors of soft tissue and undifferentiated pleomorphic sarcomas (type “MFH”) have been described in the mediastinum. In some of these cases, a definite diagnosis may not be possible in a biopsy even after exhaustive immunohistochemical stainings.
The differential diagnosis of small cell tumors includes T-lymphoblastic lymphoma, small cell carcinoma, small blue round cell sarcomas (Ewing/PNET, CIC-DUX translocated sarcomas, small cell synovial sarcoma, rhabdomyosarcoma), and neuroblastoma. Lymphomas and small cell carcinomas can be readily sorted out by expression of lymphatic and epithelial/neuroendocrine markers, respectively. CD99 staining is of limited value in the differential diagnosis, since all of these tumors including rare cases of small cell carcinomas (16) express CD99. Small cell carcinomas may be associated with paraneoplastic symptoms such as Cushing or Lambert Eaton syndrome (17).
Ewing sarcoma/PNET (“Askin tumor”) is very rare in the mediastinum and usually affects young adults. As in other anatomic locations, demonstration of a chromosomal translocation involving the EWSR1 gene is required to confirm the diagnosis (18,19). The spectrum of small blue round cell sarcomas has recently been considerably extended beyond Ewing sarcoma/PNET by the definition of tumors with CIC-DUX4 (20) and BCOR-CCNB3 gene fusions (21). However, such cases have so far not been reported in the mediastinum. Rhabdomyosarcomas are exceedingly rare in the mediastinum and are usually seen as a heterologous component of other tumors. Neuroblastomas usually occur in the posterior mediastinum in children, but a few cases have been described also in the thymus of elderly patients. In addition to the well-known MYCN gene amplifications, mutations of ALK, PHOX2B, and ATRX have been described. In addition to neuron-specific enolase (NSE), expression of GATA3 has recently been described as a valuable diagnostic marker (22).
Summary
Mediastinal soft tissue sarcomas are rare, but pose a significant diagnostic challenge due to the exceedingly large variety of other tumors with overlapping morphological and immunohistochemical features (thymomas, lymphomas and leukemias, germ cell tumors, metastases, etc.) that occur in the mediastinum. Awareness of possible differential diagnoses, close attention to the patient’s age and clinical presentation as well as an increasingly large panel of immunohistochemical antibodies and molecular tests are required for correct interpretation.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor Mirella Marino for the series “Diagnostic Problems in Anterior Mediastinum Lesions” published in Mediastinum. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med.2018.05.01). The series “Diagnostic Problems in Anterior Mediastinum Lesions” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- den Bakker MA, Marx A, Mukai K, et al. Mesenchymal tumours of the mediastinum--part II. Virchows Arch 2015;467:501-17. [Crossref] [PubMed]
- den Bakker MA, Marx A, Mukai K, et al. Mesenchymal tumours of the mediastinum--part I. Virchows Arch 2015;467:487-500. [Crossref] [PubMed]
- Carter BW, Tomiyama N, Bhora FY, et al. A modern definition of mediastinal compartments. J Thorac Oncol 2014;9:S97-101. [Crossref] [PubMed]
- Shi Q, Pan S, Bao Y, et al. Primary mediastinal myelolipoma: a case report and literature review. J Thorac Dis 2017;9:E219-25. [Crossref] [PubMed]
- Oaks J, Margolis DJ. Spindle cell lipoma of the mediastinum: a differential consideration for liposarcoma. J Thorac Imaging 2007;22:355-7. [Crossref] [PubMed]
- La Mantia E, Franco R, Rocco R, et al. Spindle cell lipoma: a rare tumor of the mediastinum. J Thorac Dis 2013;5:E152-4. [PubMed]
- Alaggio R, Coffin CM, Weiss SW, et al. Liposarcomas in young patients: a study of 82 cases occurring in patients younger than 22 years of age. Am J Surg Pathol 2009;33:645-58. [Crossref] [PubMed]
- Jo VY, Fletcher CD. p63 immunohistochemical staining is limited in soft tissue tumors. Am J Clin Pathol 2011;136:762-6. [Crossref] [PubMed]
- Lan T, Chen H, Xiong B, et al. Primary pleuropulmonary and mediastinal synovial sarcoma: a clinicopathologic and molecular study of 26 genetically confirmed cases in the largest institution of southwest China. Diagn Pathol 2016;11:62. [Crossref] [PubMed]
- Alassiri AH, Ali RH, Shen Y, et al. ETV6-NTRK3 is expressed in a subset of ALK-negative inflammatory myofibroblastic tumors. Am J Surg Pathol 2016;40:1051-61. [Crossref] [PubMed]
- Lovly CM, Gupta A, Lipson D, et al. Inflammatory myofibroblastic tumors harbor multiple potentially actionable kinase fusions. Cancer Discov 2014;4:889-95. [Crossref] [PubMed]
- Suster S, Moran CA. Spindle cell thymic carcinoma: clinicopathologic and immunohistochemical study of a distinctive variant of primary thymic epithelial neoplasm. Am J Surg Pathol 1999;23:691-700. [Crossref] [PubMed]
- Suster S, Rosai J. Thymic carcinoma. A clinicopathologic study of 60 cases. Cancer 1991;67:1025-32. [Crossref] [PubMed]
- Miettinen MM, Antonescu CR, Fletcher CDM, et al. Histopathologic evaluation of atypical neurofibromatous tumors and their transformation into malignant peripheral nerve sheath tumor in patients with neurofibromatosis 1-a consensus overview. Hum Pathol 2017;67:1-10. [Crossref] [PubMed]
- Anderson T, Zhang L, Hameed M, et al. Thoracic epithelioid malignant vascular tumors: a clinicopathologic study of 52 cases with emphasis on pathologic grading and molecular studies of WWTR1-CAMTA1 fusions. Am J Surg Pathol 2015;39:132-9. [Crossref] [PubMed]
- Zaccarini DJ, Deng X, Tull J, et al. Expression of TLE-1 and CD99 in Carcinoma: Pitfalls in Diagnosis of Synovial Sarcoma. Appl Immunohistochem Mol Morphol 2016. [Epub ahead of print].
- Zhang K, Liu W, Li Y, et al. Mediastinal small cell cancer associated with Lambert-Eaton myasthenic syndrome: A case report. Exp Ther Med 2015;10:117-20. [Crossref] [PubMed]
- Halliday J, Soon SY, Monaghan H, et al. Extraskeletal Ewing's sarcoma presenting as a mediastinal mass. Ann Thorac Surg 2010;90:1016-7. [Crossref] [PubMed]
- Manduch M, Dexter DF, Ellis PM, et al. Extraskeletal Ewing's sarcoma/primitive neuroectodermal tumor of the posterior mediastinum with t(11;22)(q24;q12). Tumori 2008;94:888-91. [Crossref] [PubMed]
- Antonescu CR, Owosho AA, Zhang L, et al. Sarcomas With CIC-rearrangements Are a Distinct Pathologic Entity With Aggressive Outcome: A Clinicopathologic and Molecular Study of 115 Cases. Am J Surg Pathol 2017;41:941-9. [Crossref] [PubMed]
- Kao YC, Owosho AA, Sung YS, et al. BCOR-CCNB3 Fusion Positive Sarcomas: A Clinicopathologic and Molecular Analysis of 36 Cases With Comparison to Morphologic Spectrum and Clinical Behavior of Other Round Cell Sarcomas. Am J Surg Pathol 2018;42:604-15. [PubMed]
- Wiles AB, Karrs JX, Pitt S, et al. GATA3 is a reliable marker for neuroblastoma in limited samples, including FNA Cell Blocks, core biopsies, and touch imprints. Cancer Cytopathol 2017;125:940-6. [PubMed]
Cite this article as: den Bakker M, Marx A, Ströbel P. The pathology of mesenchymal tumors of the mediastinum. Mediastinum 2018;2:42.


