
The role of immune checkpoint blockade for treatment of thymic epithelial tumors—a delicate balance between efficacy and side effects
Thymic epithelial tumors (TETs) are malignancies that arise from thymic epithelial cells. Despite being one of the most common anterior mediastinal primary tumors, TETs are rare. The incidence of TETs in the U.S. is 0.13 cases per 100,000 person-years based on Surveillance, Epidemiology, and End Results (SEER) cancer registries (1). TETs are categorized into thymomas and thymic carcinomas according to the World Health Organization (WHO) pathologic classification (2). Compared with thymomas, thymic carcinomas behave more aggressively with a tendency to metastasize and usually present with advanced disease (Masaoka stage III or IV) (3). The mainstay of treatment for unresectable or metastatic thymic carcinomas is platinum-based chemotherapy such as cisplatin/doxorubicin/cyclophosphamide and carboplatin/paclitaxel (4). There are no standard treatments after failure of first-line chemotherapy. Targeted therapies such as sunitinib and everolimus have some activity, but responses are usually short-lived in patients previously treated with platinum-based chemotherapy (5,6).
Immune checkpoint inhibitors (ICIs) that stimulate the immune system by blocking the inhibitory anti-programmed cell death-1 (PD-1)/programmed death ligand-1 (PD-L1) pathway have proven to be effective in a broad array of tumor types. Several studies have demonstrated that PD-L1 expression, the most widely used predictive biomarker of response to anti-PD-1/PD-L1 therapy, is high in TETs (7-10). Tumor mutations are more frequent in thymic carcinomas than in thymomas (11). In addition, thymic carcinomas are not frequently associated with autoimmune diseases such as myasthenia gravis and pure red cell aplasia that are not uncommon in patients with thymomas (4). These considerations provided the rationale for investigating the role of immune checkpoint blockade in the treatment of thymic carcinomas.
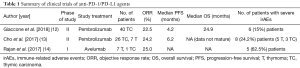
Recently, we published the results of a phase II study of pembrolizumab, an anti-PD-1 antibody, in patients with advanced refractory or recurrent thymic carcinomas (12). Patients with progressive disease who have had at least one line of chemotherapy were eligible. Those with a history of autoimmune disorders were excluded. Study treatment consisted of pembrolizumab 200 mg intravenously every 3 weeks up to 2 years. The primary endpoint was objective response rate (ORR) with secondary endpoints of progression-free survival (PFS) and overall survival (OS). A total of 40 patients were enrolled and treated with pembrolizumab. The majority of the patients (97.5%) had stage IV disease. The median number of prior lines of systemic therapy was 2 (range, 1–6). The ORR was 22.5% (95% CI: 10.8–38.5) with 1 (3%) patient achieving a complete response. Twenty-one (53%) patients had stable disease. Responses were rapid and durable with median time to response of 6 weeks (range, 6–24 weeks) and median duration of response of 22.4 months (95% CI: 12.3–34.7 months), respectively. Median PFS and OS were 4.2 months (95% CI: 2.9–10.3 months) and 24.9 months (95% CI: 15.5–not reached), respectively. Pre-treatment tumor samples were analyzed to determine whether PD-L1 expression and an 18-gene T-cell-inflamed interferon-gamma signature are predictive of treatment outcomes. In 10 patients with high PD-L1 expression, defined as positive staining in at least 50% of tumor cells, 6 (60%) achieved a response. On the contrary, among 27 patients with PD-L1 expression less than 50%, only 3 (11%) patients achieved a response. Expression of the interferon-gamma signature was also significantly higher in responders than in non-responders. In addition, there were significant correlations between PFS and OS, and PD-L1 expression and the interferon-gamma signature.
While the treatment was well tolerated with most adverse events (AEs) being grade 1 or 2, it should be noted that 6 (15%) patients developed one or more severe immune-related AEs (irAEs). Severe irAEs included hepatitis, myocarditis, polymyositis, bullous pemphigoid, diabetes mellitus type 1, and myasthenia gravis. Severe irAEs were resolved with treatment interruption and immunosuppressant agents, mainly corticosteroids. There was no treatment-related death. Two patients who developed myocarditis and myositis underwent placement of pacemaker. Notably, in one patient who developed myocarditis and myositis, a T-cell clone which was present in pre-treatment blood increased in post-treatment blood, and was also found in tumor and muscle biopsy samples, raising the speculation that an epitope shared between tumor and normal tissue might have been responsible for the development of myocarditis.
A few other studies have reported the safety and efficacy of anti-PD-1/PD-L1 therapy for the treatment of TETs (Table 1). A phase II trial of pembrolizumab in Korean patients with TETs (7 thymomas, 26 thymic carcinomas) whose disease progressed after platinum-containing chemotherapy showed a comparable ORR of 23.1% in patients with thymic carcinoma and an ORR of 28.6% in patients with thymomas (13). Patients with a history of active autoimmune disease requiring systemic treatment within the past 1 year were excluded, but 8 (24.2%) patients (5 thymomas, 3 thymic carcinomas) experienced severe irAEs resulting in treatment discontinuation. A trial of avelumab, an anti-PD-L1 antibody, in patients with TETs (7 thymomas, 1 thymic carcinoma) showed 2 (25%) confirmed partial responses (14). Severe irAEs were observed in 5 (62.5%) patients, three of which were myocarditis.
Full table
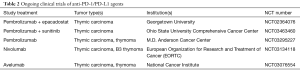
In summary, anti-PD-1/PD-L1 therapy has anticancer activity against TETs with ORRs ranging between 20–25%. Durable responses observed in responders make anti-PD-1/PD-L1 blockade an attractive therapeutic approach in this patient population. The use of anti-PD-1/PD-L1 therapy for the treatment of TETs is associated with higher frequency of irAEs. While most irAEs were manageable with immunosuppressant agents such as corticosteroids, early detection of irAEs through careful monitoring of patients is necessary. Several ongoing studies including a study of pembrolizumab and epacadostat, an indoleamine 2,3-dioxygenase-1 (IDO1) inhibitor at our institution (NCT02364076) will shed further light on the safety and efficacy of immune check point therapy in TETs (Table 2). Future research should concentrate on developing more effective immunotherapy strategies and identifying biomarkers of response and toxicity to improve outcomes in patients suffering from TETs.
Full table
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors Mirella Marino and Brett W. Carter for the series “Dedicated to the 8th International Thymic Malignancy Interest Group Annual Meeting (ITMIG 2017)” published in Mediastinum. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med.2018.04.05). The series “Dedicated to the 8th International Thymic Malignancy Interest Group Annual Meeting (ITMIG 2017)” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Engels EA. Epidemiology of thymoma and associated malignancies. J Thorac Oncol 2010;5:S260-5. [Crossref] [PubMed]
- Marx A, Strobel P, Badve SS, et al. ITMIG consensus statement on the use of the WHO histological classification of thymoma and thymic carcinoma: refined definitions, histological criteria, and reporting. J Thorac Oncol 2014;9:596-611. [Crossref] [PubMed]
- Liu HC, Hsu WH, Chen YJ, et al. Primary thymic carcinoma. Ann Thorac Surg 2002;73:1076-81. [Crossref] [PubMed]
- Kelly RJ, Petrini I, Rajan A, et al. Thymic malignancies: from clinical management to targeted therapies. J Clin Oncol 2011;29:4820-7. [Crossref] [PubMed]
- Thomas A, Rajan A, Berman A, et al. Sunitinib in patients with chemotherapy-refractory thymoma and thymic carcinoma: an open-label phase 2 trial. Lancet Oncol 2015;16:177-86. [Crossref] [PubMed]
- Zucali PA, De Pas T, Palmieri G, et al. Phase II Study of Everolimus in Patients With Thymoma and Thymic Carcinoma Previously Treated With Cisplatin-Based Chemotherapy. J Clin Oncol 2018;36:342-9. [Crossref] [PubMed]
- Arbour KC, Naidoo J, Steele KE, et al. Expression of PD-L1 and other immunotherapeutic targets in thymic epithelial tumors. PLoS One 2017;12:e0182665 [Crossref] [PubMed]
- Katsuya Y, Fujita Y, Horinouchi H, et al. Immunohistochemical status of PD-L1 in thymoma and thymic carcinoma. Lung Cancer 2015;88:154-9. [Crossref] [PubMed]
- Padda SK, Riess JW, Schwartz EJ, et al. Diffuse high intensity PD-L1 staining in thymic epithelial tumors. J Thorac Oncol 2015;10:500-8. [Crossref] [PubMed]
- Yokoyama S, Miyoshi H, Nakashima K, et al. Prognostic Value of Programmed Death Ligand 1 and Programmed Death 1 Expression in Thymic Carcinoma. Clin Cancer Res 2016;22:4727-34. [Crossref] [PubMed]
- Petrini I, Meltzer PS, Kim IK, et al. A specific missense mutation in GTF2I occurs at high frequency in thymic epithelial tumors. Nat Genet 2014;46:844-9. [Crossref] [PubMed]
- Giaccone G, Kim C, Thompson J, et al. Pembrolizumab in patients with thymic carcinoma: a single-arm, single-centre, phase 2 study. Lancet Oncol 2018;19:347-55. [Crossref] [PubMed]
- Cho J, Ahn MJ, Yoo KH, et al. A phase II study of pembrolizumab for patients with previously treated advanced thymic epithelial tumor. J Clin Oncol 2017;35:8521.
- Rajan A, Heery C, Mammen A, et al. OA18.03 Safety and Clinical Activity of Avelumab (MSB0010718C; Anti-PD-L1) in Patients with Advanced Thymic Epithelial Tumors (TETs). J Thorac Oncol 2017;12:S314-5. [Crossref]
Cite this article as: Kim C, Giaccone G. The role of immune checkpoint blockade for treatment of thymic epithelial tumors—a delicate balance between efficacy and side effects. Mediastinum 2018;2:39.


