
Breakthroughs in thymic malignancies using international collaborative data
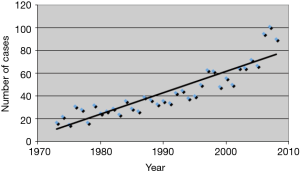
Thymic epithelial tumors (TET) are rare with an overall incidence of 0.15 per 100,000, however, the incidence appears to be increasing (1) (Figure 1). Like many rare tumors, there has been a paucity of data from which to derive best practices, leading to the development of numerous muticenter large databases. These efforts allow for pooling of data and better characterization of the biology, clinical characteristics, and patient outcomes of thymic tumors.
In recent years, the Japanese Association for Research on the Thymus (JART), European Society of Thoracic Surgeons (ESTS), and Chinese Alliance for Research in Thymoma (ChART) have all developed data sets that have significantly contributed to our understanding of thymic tumors. The recent report from the International Thymic Malignancy Interest Group Retrospective Database (ITMIG) is the largest such effort to date, including 7,795 cases of TET from 56 institutions from Asia, Europe, and North America. While the data is retrospective and does not include pathologic review for verification, it does outline patient demographics and treatments, along with pathologic staging, and patient outcomes.
The coexistence of paraneoplastic autoimmune (PN/AI) syndromes and thymic tumors is well established. Most large studies agree that about 30–40% of patients with TET will have a paraneoplastic syndrome (2-6), most commonly myasthenia gravis (MG). Up to 10–20% of patients with MG have a thymoma while 30% of patients with thymoma have or will develop MG suggesting shared epigenetics between the two diseases. This shared epigenetics may explain why thymectomy has been shown to benefit patients with MG even in the absence of a thymic tumor (7).
Padda et al. set out to characterize the clinical characteristics and treatment strategies used in patients with PN/AI syndromes in the ITMIG retrospective database and clarify the prognostic role of PN/AI in patients with TET. Using this pooled data set, the authors were able to include the 6,297 cases from 1951–2012.
Consistent with other retrospective data series, the ITMIG cohort contained a large proportion of thymoma (86%). Interestingly, 12% of the cohort had thymic carcinoma and 2% had neuroendocrine tumors (NETT). The authors also found other PN syndromes: pure red cell aplasia and hypogammaglobulinemia. Unsurprisingly, patients with PN/AI tended to have earlier stage tumors, with 99% of the patients in the registry undergoing surgical resection.
Significant differences were seen in nearly all the clinical characteristics between patients with and without PN/AI syndromes. PN/AI+ patients tended to be younger (50 vs. 55), female, and European, while patients without PN/AI were more likely to be male and from Asia. The WHO histotype B2 occurred more frequently in patients with PN/AI+, while PN/AI (−) tended to have AB histotype and a much higher incidence of thymic carcinoma in PN/AI (−) patients (17% vs. 2%). The authors also noted a higher incidence of total thymectomy with complete R0 resection in patients with PN/AI (+).
The authors note a significantly lower cumulative incidence of recurrence in the PN/AI (+) cohort overall however when correcting for subgroup, thymic carcinoma, and stage they noted no difference. When the authors looked at overall survival (OS) the significant survival benefit for PN/AI (+) remained even within the thymoma and thymic carcinoma subgroups. Within the thymic carcinoma group, the survival benefit appeared to be due to differences in Stage III–IVB subgroups. When the authors reviewed survival within 3 times periods, the authors noted a significant difference in the proportion of PN/AI (+) within each period and a significant survival benefit in the most recent time.
On multivariate analysis the authors noted significantly higher recurrence from older age, histology of thymic carcinoma and NETT, stage III–IVB disease, larger tumor size, and R2 resection. Characteristics associated with decreased recurrence included: Asia patients and curative radiation. The authors noted worse OS for patients that received curative chemotherapy as well as the factors affecting recurrence. For improved OS the authors noted curative radiation and B1 histotype. In all the multivariate analyses PN/AI (+) was not independently associated with recurrence-free survival (RFS) or OS.
The authors concluded that PN/AI (+) was associated with favorable prognostic factors including younger age, female sex, lower incidence of thymic carcinoma, earlier stage disease, complete R0 resection, and type B2 pathology.
As noted, there is a significant amount of confounding in the ITMIG data. This data is retrospective. Furthermore, data in the ITMIG is dependent on local clinicians, patients, and pathologic review. While there are recommendations for surveillance currently, it is hard to assess if all providers are following these recommendations. In the United States, this issue is pronounced. Large proportions of society live in rural areas with minimal access to advanced healthcare and there are very few regional centers. Thus, the predominance of care can occur in areas poorly equipped to treat patients with thymic tumors.
All of the pooled data sets are limited by the past. As noted by the authors the incidence of PN/AI within the data appears to be decreasing over time. While this could represent a true occurrence, it is more likely that patients with PN/AI (−) tumors were not diagnosed with TET during previous generations as imaging and pathologic criteria lagged behind.
I want to congratulate the authors on the breath and scope of their study. They have been able to mine a large amount of data and distinguish important points that bare further investigation. The ITMIG retrospective database is the largest international database collecting data on TET. Researchers now have access to a large number of data sets regarding thymic tumors (JART, ChART, ESTS, and ITMIG). We suspect as these pooled data sets become more robust and complete, small differences between tumors will be identified and can lead to better understanding of the pathophysiology of TETs.
The fact that PN/AI is not predictive of disease-free survival (DFS) or OS in this large cohort seems to indicate that it is more of a symptom that allows for earlier diagnosis and treatment. However, this idea does not seem to hold up when the other findings are evaluated. PN/AI (+) seems to occur in females, Europeans, and with WHO B2 pathology. The current theories linking PN/AI (+) to TET seems to suggest that PN/AI (+) is a different disease then PN/AI (−). This difference is crucial to understand and may hold a key to improved treatments for all TET patients.
I ask the authors if there may be reporting differences between countries that may cause a skewing of the data? There is a significant difference between European TET and Asian TET. This is not just concerning PN/AI status but also with thymic carcinoma. Like with gastric cancer, regional differences may have a profound effect on treatment strategies and outcomes. Again, this points to the lack of understanding of tumorigenesis and the biological activities of varying TETs.
We will add a note of caution with regard to comparing data from the different data sets. As noted by the authors, there is some data crossover between these differing collections. As such, it becomes imperative that researchers do not allow corruption of data by over representation. I hope that in the coming years the different data sets can be combined into a large collection with an increasing amount of tumor biology and pathologic consistency.
Like with other rare tumors, the great advances in TET must occur in a retrospective fashion. However, like in much of oncology the ability to store live tumor after resection in tissue banks is the only way to improve treatment strategies and outcomes as new science is discovered.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by Section Editor Xiaomin Niu (Department of Shanghai Lung Cancer Center, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med.2018.03.24). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Helm JM, Lavy D, Figueroa-Bodine J, et al. Metastatic Malignant Thymoma to the Abdomen: A SEER Database Review and Assessment of Treatment Strategies. World J Oncol 2017;8:147-50. [Crossref] [PubMed]
- Evoli A, Lancaster E. Paraneoplastic disorders in thymoma patients. J Thorac Oncol 2014;9:S143-7. [Crossref] [PubMed]
- Marx A, Pfister F, Schalke B, et al. The different roles of the thymus in the pathogenesis of the various myasthenia gravis subtypes. Autoimmun Rev 2013;12:875-84. [Crossref] [PubMed]
- Hirokawa M, Sawada K, Fujishima N, et al. Long-term response and outcome following immunosuppressive therapy in thymoma-associated pure red cell aplasia: a nationwide cohort study in Japan by the PRCA collaborative study group. Haematologica 2008;93:27-33. [Crossref] [PubMed]
- Montella L, Masci AM, Merkabaoui G, et al. B-cell lymphopenia and hypogammaglobulinemia in thymoma patients. Ann Hematol 2003;82:343-7. [Crossref] [PubMed]
- Bernard C, Frih H, Pasquet F, et al. Thymoma associated with autoimmune diseases: 85 cases and literature review. Autoimmun Rev 2016;15:82-92. [Crossref] [PubMed]
- Wolfe GI, Kaminski HJ, Aban IB, et al. Randomized Trial of Thymectomy in Myasthenia Gravis. N Engl J Med 2016;375:511-22. [Crossref] [PubMed]
Cite this article as: Joseph S, Matthew Helm J, Edwards M. Breakthroughs in thymic malignancies using international collaborative data. Mediastinum 2018;2:32.


