Paraneoplastic neurological syndromes associated with mediastinal tumors
Introduction
Mediastinal masses are mostly found in the anterior compartment and include several pathological entities. Tumors of the anterior mediastinum are more likely to be malignant, and more frequently comprise thymoma, lymphoma and germ cell tumors (1).
Clinical presentation of a mediastinal mass may be related to the compression or invasion of the adjacent structures leading, in some cases, to life-threatening emergencies. In other patients, these tumors may be heralded by non-local symptoms caused by paraneoplastic neurological diseases (PND). By definition, these disorders are not related to tissue invasion by the tumor, distant metastases or metabolic/toxic effects of cancer therapy. Rather, they are thought to be triggered by an autoimmune response to antigens co-expressed by the tumors and the target organs, i.e. the nervous system and the muscle tissue (2). In addition, a broad range of non-neurological PDs can occur in association with thymoma. The recognition of a PD can be critical for tumor diagnosis and may predict a specific type of cancer.
Here we will focus on the PND associated with thymoma, lymphoma and thoracic germ cell cancer.
The role of thymoma in autoimmunity
Immunological tolerance can be defined as the state of unresponsiveness to a self or non-self antigen. The thymus is the central organ of immunologic tolerance. Progenitor T cells migrate from the bone marrow to the thymus to maturate and differentiate through interactions with cortical and medullary thymic epithelial cells (TECs). In this tightly regulated process T cells undergo positive and negative selection, and the cells with high reactivity to self-antigens (potential cause of autoimmunity) are eliminated (3). Thymomas originate from TECs and are distinct into histological subtypes according to the W.H.O. classification published in 1999 (4) and modified in 2004 (5).
The pathogenic mechanisms of thymoma-associated autoimmunity have been investigated in relation to myasthenia gravis (MG). Several abnormalities have been described in the molecular biology and immunocytochemistry of thymoma that can alter T-cell development. In MG-associated thymoma, neoplastic epithelial cells still express epitopes of the acetylcholine receptor (AChR) subunits and titin, but also show a decreased expression of the major histocompatibility complex (MHC) class II molecules, which are required for positive selection of T lymphocytes. Most thymoma neoplastic cells also fail to express the Autoimmune Regulator (AIRE) transcription factor, which is critical for T cell negative selection. Moreover, it has been shown that the production of regulatory T-lymphocytes, which suppress the immune response toward self antigens are diminished in thymomas (6).
PND associated with thymoma
The neurologists’ interest in thymoma was initially stirred by its association with MG. How this rare, slow-growing tumor came to be involved in the pathogenesis of a prototypical autoimmune disease has long been a matter of debate. Later, it became evident that thymoma was central to a broader disease spectrum and what we learnt from paraneoplastic MG paved the way to the understanding of the mechanisms of PND of the peripheral and central nervous system (CNS). All these conditions, as well as non-neurological thymoma-associated PDs, can also occur as idiopathic non- paraneoplastic diseases.
Disorders of the neuro-muscular transmission
Myasthenia Gravis
MG is the most common thymoma-associated disease, as up to 40% of individuals with thymoma develop MG, while 15–20% of MG patients are diagnosed with thymoma.
MG is a disorder of the neuromuscular junction, caused by antibodies (Abs) against extracellular determinants of postsynaptic proteins. The antibody attack results, through different mechanisms, in morphological and functional alterations leading, in turn, to the impairment of the neuromuscular transmission.
The clinical hallmark of MG is fluctuating muscle weakness, worsened by exertion and relieved by rest. The more common involvement of certain muscle groups (extrinsic ocular, facial, oropharyngeal, neck, limb proximal muscles) accounts for a typical clinical picture in most cases. On the other hand, weakness severity shows remarkable variability, from purely ocular symptoms to severe generalized disease. Weakness of respiratory muscles can lead to respiratory failure (so-called myasthenic crisis) requiring assisted ventilation (7). Around 90% of MG patients have serum Abs to the AChR. These Abs induce severe alterations of the post-synaptic membrane through complement activation and increased AChR degradation, and, to a lesser extent, by interfering with the ACh binding site (8). MG with AChR Abs (AChR-MG) is frequently associated with thymus alterations, such as follicular hyperplasia and thymoma, both playing a role in the disease pathogenesis (9).
In anti-AChR negative patients, Abs to other synaptic proteins (such as the muscle-specific tyrosine kinase receptor, MuSK, and the low-density lipoprotein receptor-related protein 4, LRP4) can be detected and identify distinct disease subtypes (10). The MuSK-LRP4-Agrin complex is essential for the neuromuscular junction formation and its maintenance in adult life. MuSK, activated by the nerve secreted agrin through its co-receptor LRP4, promotes AChR clustering (11), and LRP4 delivers retrograde signals contributing to presynaptic differentiation (12). Five to seven percent of anti-AChR negative patients have Abs to MuSK, serum Abs to LRP4 can be found in a low proportion of anti-AChR and -MuSK negative cases and, more recently, serum immunoglobulin G (IgG) to agrin have been reported (10). In addition, a few patients with clinical and electrophysiological signs of MG do not have detectable Abs (seronegative MG). Currently, there are no data supporting a pathogenic link with the thymus for all these forms of MG.
Despite isolated cases of thymoma have been described in MuSK-MG (13), LRP4-MG (14) and in the seronegative disease (15), the association with thymoma is essentially restricted to AChR-MG.
Clinical and pathogenic aspects of thymoma-associated AChR-MG
In the great majority of patients, the onset of MG leads to thymoma detection, as a study of the mediastinum by computed tomography scan is routinely performed upon the neurological diagnosis. More rarely, MG presentation occurs months, and even years, after thymoma treatment and can be a sign of tumor recurrence.
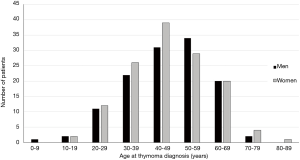
In MG patients, the risk of having a thymoma is low in the first two decades and after 70 years of age; it increases in between, with a peak incidence in the fourth through the sixth decade. Figure 1 shows the age at thymoma diagnosis in 256 MG patients seen in our institution between 1980 and 2016. As shown in the figure, there was no gender bias in our population. This finding has been reported in other thymoma-associated MG cohorts (16,17).
Thymoma-associated MG is generally severe, with a high rate of life-threatening symptoms, and patients tend to remain dependent on immunosuppressive treatment. A broad range of other neurological and non-neurological PDs can occur in these cases, both before and after tumor treatment. The onset of any of these disorders as well as a serious worsening of MG symptoms may predict a tumor recurrence and should prompt specific imaging studies.
Patients with AChR-MG often have serum Abs to the giant muscle protein titin and the ryanodine receptor (RyR). These Abs, formerly called anti-striational Abs, are strongly associated with MG-related thymoma (anti-titin are positive in 95% and anti-RyR Abs in 70% of these patients), are detected also in nearly 50% of late-onset non-thymoma cases, while are very uncommon in early-onset MG (18,19). As anti-striational Abs target intracellular antigens their pathogenicity is uncertain, though they are valuable markers of thymoma, at least in young MG patients (20).
Kv1.4 Abs that target the alpha subunit of the muscle voltage-gated potassium channel (VGKC) were reported in 12–15% of Japanese MG patients, in association with thymoma, severe MG and myocarditis with arrhythmias (21,22). Interestingly, these findings were not confirmed in Caucasian patients (23).
Thymoma-associated MG is thought to be caused by defects of central tolerance related to the neoplastic thymus microenvironment. Similar mechanisms may be operating in other PDs. As mentioned before, in the normal thymus, developing T lymphocytes go through sequential maturations stages from CD4+CD8+ double positive (DP) cells in the thymic cortex, to CD4+CD8− and CD4−CD8+ single positive (SP) T cells in the medulla. Positive and negative selection processes secure that (mostly) self-tolerant SP T cells are released into the periphery. Stromal cells, such as TECs and dendritic cells are crucial to the shaping of T lymphocyte repertoire (24).
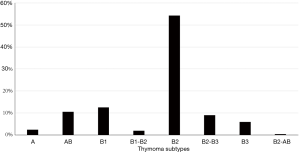
Thymoma subtypes differ in their thymopoietic capacity (22). AB and B thymomas are able to support thymocyte development and harbor a high number of DP cortical T cells. Conversely, thymopoietic function is very low in A thymoma and nearly absent in thymic carcinoma (16).
Figure 2 shows thymoma histological subtypes in our population. All thymoma specimens were reviewed over the time by the local pathologists and classified according to the WHO system (4,5).
B thymomas are the most common subtypes related to MG (see Figure 2), and both intratumorous T lymphocyte maturation and export of mature T cells appear to be prerequisites for paraneoplastic MG development (16). Although neoplastic TECs express single epitopes of AChR subunits and the protein titin, thymoma tissue has a low expression of MHC class II molecules and AIRE gene, both required for an effective thymocyte selection (17). Defective medullary function in “cortical” B thymomas (that have sparse medullary areas) can be responsible for the export of autoreactive CD4+ SP T cells and for a reduced production of T regulatory cells. Such unbalance appears to be a distinctive feature of MG-associated thymomas (17).
Protein tyrosine phosphatase non-receptor 22 (PTPN22) and cytotoxic T lymphocyte associated antigen 4 (CTLA-4) are both inhibitors of T lymphocyte activation. Variants of these genes were reported in association with different autoimmune diseases, including MG (25,26). CTLA-4+49A>G (27,28) and two different PTPN22 genotypes (28,29) were found to be associated with MG and thymoma. In addition, peripheral and intra-tumorous T cells from MG thymoma patients had higher expression levels of the anti-apoptotic factor cellular FLICE-like inhibitory protein (c-FLIP) than non-MG thymomas (30). These results confirm the role of activated T cells in the pathogenesis of thymoma-associated autoimmune diseases.
Few patients with MG may have a thymolipoma, a rare benign tumor of the thymus containing mature adipose tissue and thymic remnants. In these cases, MG is generally anti-AChR positive, and its severity and outcome do not seem to differ from those of the thymoma associated disease (31,32).
Lambert-Eaton myasthenic syndrome (LEMS)
LEMS is a rare disorder of the neuromuscular ad autonomic transmission, in which Abs to the voltage-gated calcium channel (VGCC) at the nerve terminal markedly reduce acetylcholine release. These patients complain of muscle weakness typically prevalent on leg proximal muscles, loss of tendon reflexes, and autonomic disturbances (33). Paraneoplastic LEMS is generally related to small-cell lung cancer (33), but the association with thymic tumors has rarely been reported (34,35). In these patients, oncologic treatment resulted in marked clinical benefit.
Disorders of peripheral and CNS
Acquired neuromyotonia (aNMT) and the Morvan’s syndrome (MoS) are well characterized disorders due to peripheral nerve hyperexcitability. These conditions, when paraneoplastic, are generally associated with thymoma. The earliest evidence for NMT to be an antibody-mediated disease derived from studies of passive transfer of disease-related electrophysiological abnormalities to mice with patients’ IgG. Then, NMT and MoS have been recognized as autoimmune channelopathies since the identification of autoAbs binding to the VGKCs (36).
These Abs were originally detected with a radioimmunoassay in which VGKCs solubilized from mammalian brain membranes and labelled with iodinated-dendrotoxin were precipitated by patients’ IgG. The Abs were believed to bind directly to alpha dendrotoxin-bound VGKC subunits (Kv 1.1, 1.2 and 1.6). However, it has recently been shown that patients’ serum IgG most frequently bind to the extracellular domains of leucine rich glioma inactivated protein 1 (LGI1) and contactin-associated protein-like 2 (CASPR2), two proteins complexed with VGKCs (37,38). Indeed, CASPR2 Abs are more commonly detected in patients with NMT and MoS associated with thymoma (39), while LGI1 Abs are usually found in patients with non-paraneoplastic autoimmune encephalitis (38). In addition, Abs to the Netrin-1 receptors (DCC and UNC5A) have lately been reported in NMT or MoS occurring in patients with thymoma-associated MG (40). The expression of DCC, UNC5A, and Caspr2 proteins was demonstrated both in thymoma samples and normal thymus (40). The pathogenic role of the Netrin-1 receptor Abs has not been investigated.
Acquired neuromyotonia
aNMT is characterized by spontaneous muscle activity due to continuous nerve depolarization. Clinical presentation includes myokymia (diffuse muscle twitching), fasciculations and cramps; hyperhidrosis is common and patients with long-standing disease can develop muscle hypertrophy (41). Electromyography shows spontaneous motor unit discharges as doublet, triplet, multiplet bursts (myokymic discharges), and, less commonly, longer bursts with high intraburst frequency (neuromyotonic discharges) (42). In up to 20% of cases aNMT is associated with signs of CNS involvement, such as mood changes and sleep disturbance. aNMT generally occur in association with MG in patients with thymoma. Importantly, both the onset and worsening of aNMT can herald a tumor recurrence (43).
NMT symptoms usually improve with sodium channel-blockings as carbamazepine, phenytoin or lamotrigine. In patients with severe symptoms plasma-exchange and immunosuppression with corticosteroids and azathioprine can be required (44). When associated with thymoma, oncologic treatment can significantly improve the neurological diseases.
Morvan’s syndrome
In patients with MoS, NMT is associated with encephalopathy, sleep disorder with prolonged periods of insomnia (agrypnia), dysautonomia (hyperhidrosis, sphincter dysfunction), and/or pain. CASPR2 Abs are most frequently detected in these patients, often in association with LGI1 Abs. A thymoma can be detected in up to 50% of patients with CASPR2 Abs, generally in association with MG (45).
Both peripheral and central symptoms respond to plasma-exchange and immunosuppressive treatment, and may improve further with thymoma treatment.
Encephalitis
Paraneoplastic encephalitis usually presents with subacute (days to weeks) memory impairment associated with seizures and mood or behavioral disturbances (46).
Some patients develop hallucinations, delusions, and bizarre behavior at the onset of their illness and may initially be referred to a psychiatrist. Seizures and alterations in the level of consciousness ensue in most cases. Brain MRI typically shows hyperintense lesions of the medial temporal lobes on T2-weighted images, but can otherwise be negative. Cerebrospinal fluid (CSF) analysis may detect increased protein content or moderate lymphocytic pleocytosis, and in a minority of patients may show the presence of oligoclonal bands. Thymoma-associated autoimmune encephalitis is generally associated with pathogenic autoAbs binding to membrane neural antigens and often responds favorably to immunotherapy and anti-neoplastic therapy. The presence of Abs to intracellular antigens is rarer.
Serum IgG specific for the extracellular domain of the VGKC-complex proteins LGI1 and CASPR2 are the most commonly Abs detected in patients with thymoma-associated encephalitis. In particular, while LGI1 Abs are mostly detected in non-paraneoplastic encephalitis, patients with thymoma more often have Abs binding to CASPR2 or to both LGI1 and CASPR2 (Figure 3). In a recent analysis performed in 256 patients with LGI1-IgG and/or CASPR2-IgG a thymoma was detected in 33% of patients with both LGI1 and CASPR2-Abs, in 5% of patients with isolated CASPR2- and in less than 1% of patients with isolated LGI1 Abs (47).
Other neuronal autoAbs binding to neuronal membrane proteins detected in patients with autoimmune encephalitis and thymoma are specific to AMPA (AMPAR) and GABAA (GABAAR) receptors. Encephalitis associated with AMPAR-IgG usually presents like classical limbic encephalitis with memory disturbances, alteration of mood and seizures. In up to 40% of patients with AMPAR-IgG a diffuse encephalopathy can be observed, while a minority of patients can develop a different syndrome, such as rapidly progressive dementia or psychosis. Patients with GABAAR-IgG usually develop seizures refractory to standard anti-epileptic therapy (48). MRI may reveal multifocal cortical and juxta-cortical hyperintense lesions on T2-weighted images. Most patients respond to immunotherapy. Thymoma has been detected in around 30% of adult patients with GABAAR Abs (49).
AutoAbs binding to the collapsin response mediator protein 5 (CRMP5), also known as CV2, an intracellular protein involved in the regulation of dendritic development and synaptic plasticity, can be observed in patients with thymoma and encephalitis. These patients can present with limbic encephalitis or chorea and immunotherapy is often beneficial (50,51)
Inflammatory muscle diseases
Different autoimmune muscle diseases, such as dermatomyositis (52), granulomatous myositis (53) and polymyositis (54) have been reported in association with thymic tumors, with and without MG. All these disorders manifest with muscle weakness, myalgia, increased serum levels of muscle enzymes and abnormal hyperintense on STIR MRI. Cardiac muscle may be involved with heart failure and arrhythmias (54). In these cases, a role for anti-striational Abs has been suggested (21) but it remains uncertain owing to the lack of systematic studies.
PND associated with lymphoma
PND are a rare accompaniment of Hodgkin lymphoma (HL) and non-HLs (NHLs) (55). Paraneoplastic cerebellar degeneration (PCD) is the most frequent PND in patients with HL (56). PCD represents a classical PND and occurs most frequently in patients with ovarian, breast and small cell lung cancer or HL, while only few cases of PCD associated with NHL have been reported to date (57). PCD patients usually develop dizziness and vertigo that rapidly progress to severe ataxia, dysarthria, diplopia, and nystagmus. CSF examination can reveal increased protein concentration or mild pleocytosis, and the MRI studies are initially normal while cerebellar atrophy can be observed later in the course of the disease. The symptoms of PCD may precede the diagnosis of HL in up to 80% of patients (58). The most common autoAb detected in patients with PCD and HL are Tr-IgG that bind to the Delta and Notch-like epidermal growth factor-related receptor (DNER) (59), a membrane protein highly expressed in the dendrites of Purkinje cells that is critical for the development of the cerebellum.
IgG specific for the metabotropic glutamate receptor 1 (mGluR1) were originally identified in two patients with PCD and HL. To date 16 patients with mGluR1 Abs have been described and 6 of them had a history of hematologic malignancy (HL, 2; NHL, 1; T-cell cutaneous lymphoma, 2; acute lymphocytic leukemia, 1) (60-63).
Paraneoplastic neurological syndromes associated with mediastinal germ cell tumors
Mediastinal germ cell cancers are rare and heterogeneous. In a retrospective multicenter study performed in France to establish the frequency and the prognosis of primary mediastinal germ cell tumors, non-seminomatous germ cell tumors (NSGT) were detected in more than 60% of the patients, followed by seminomas observed in 32%, while thoracic teratomas represented the most rare cancer types (64).
Few patients with paraneoplastic encephalitis associated with Abs binding to Ma2 or to NMDA receptor (NMDAR) and mediastinal germ cell tumor have been reported to date.
Encephalitis with anti-Ma2 Abs may be associated with seminoma or NSGT. Patients with Ma2-IgG usually develop a limbic encephalitis associated with symptoms due to diencephalic involvement (e.g., hyperthermia, narcolepsy and syndrome of inappropriate antidiuresis) or brainstem dysfunction while some patients can present with symptoms of brainstem dysfunction (e.g., ataxia, diplopia, dysphagia, dysarthria) (65).
Anti-NMDAR encephalitis has been rarely reported in patients, mainly males, with thoracic teratoma. This form of autoimmune encephalitis has characteristic clinical features. Patients develop behavioral disturbances and then seizures often refractory to standard anti-epileptic therapy and movement disorders (oro-buccal dyskinesias and dystonic postures) (66). Coma, central hypoventilation and dysautonomia can occur in the course of the disease leading to patient admission to ICU or even to patient death if appropriate treatment is not performed. There are no standardized guidelines for the treatment of patients with anti-NMDAR encephalitis. Plasma exchange, intravenous immunoglobulin and high dose intravenous steroids associated with cancer removal can result in the improvement of the neurological deficits (67). Rituximab or cyclophosphamide can be efficacious treatments for patients not responding to first-line therapies.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor Mirella Marino for the series “Diagnostic Problems in Anterior Mediastinum Lesions” published in Mediastinum. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med.2018.01.03). The series “Diagnostic Problems in Anterior Mediastinum Lesions” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Duwe BV, Sterman DH, Musani AI. Tumors of the mediastinum. Chest 2005;128:2893-909. [Crossref] [PubMed]
- Hoftberger R, Rosenfeld MR, Dalmau J. Update on neurological paraneoplastic syndromes. Curr Opin Oncol 2015;27:489-95. [Crossref] [PubMed]
- Klein L, Kyewski B, Allen PM, et al. Positive and negative selection of the T cell repertoire: what thymocytes see (and don't see). Nat Rev Immunol 2014;14:377-91. [Crossref] [PubMed]
- Rosai J, Sobin LH, World Health Organization. Histological typing of tumours of the thymus. In: International histological classification of tumours. Berlin: Springer, 1999.
- WHO Classification of Tumors. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. Lyon: International Agency for Research on Cancer (IARC) Press, 2004.
- Marx A, Willcox N, Leite MI, et al. Thymoma and paraneoplastic myasthenia gravis. Autoimmunity 2010;43:413-27. [Crossref] [PubMed]
- Keesey JC. Clinical evaluation and management of myasthenia gravis. Muscle Nerve 2004;29:484-505. [Crossref] [PubMed]
- Gomez AM, Van Den Broeck J, Vrolix K, et al. Antibody effector mechanisms in myasthenia gravis-pathogenesis at the neuromuscular junction. Autoimmunity 2010;43:353-70. [Crossref] [PubMed]
- Marx A, Pfister F, Schalke B, et al. The different roles of the thymus in the pathogenesis of the various myasthenia gravis subtypes. Autoimmun Rev 2013;12:875-84. [Crossref] [PubMed]
- Gilhus NE. Myasthenia Gravis. N Engl J Med 2016;375:2570-81. [Crossref] [PubMed]
- Burden SJ, Yumoto N, Zhang W. The role of MuSK in synapse formation and neuromuscular disease. Cold Spring Harb Perspect Biol 2013;5:a009167 [Crossref] [PubMed]
- Yumoto N, Kim N, Burden SJ. Lrp4 is a retrograde signal for presynaptic differentiation at neuromuscular synapses. Nature 2012;489:438-42. [Crossref] [PubMed]
- Evoli A, Alboini PE, Bisonni A, et al. Management challenges in muscle-specific tyrosine kinase myasthenia gravis. Ann N Y Acad Sci 2012;1274:86-91. [Crossref] [PubMed]
- Marino M, Scuderi F, Samengo D, et al. Flow Cytofluorimetric Analysis of Anti-LRP4 (LDL Receptor-Related Protein 4) Autoantibodies in Italian Patients with Myasthenia Gravis. PLoS One 2015;10:e0135378 [Crossref] [PubMed]
- Maggi L, Andreetta F, Antozzi C, et al. Two cases of thymoma-associated myasthenia gravis without antibodies to the acetylcholine receptor. Neuromuscul Disord 2008;18:678-80. [Crossref] [PubMed]
- Okumura M, Fujii Y, Shiono H, et al. Immunological function of thymoma and pathogenesis of paraneoplastic myasthenia gravis. Gen Thorac Cardiovasc Surg 2008;56:143-50. [Crossref] [PubMed]
- Marx A, Porubsky S, Belharazem D, et al. Thymoma related myasthenia gravis in humans and potential animal models. Exp Neurol 2015;270:55-65. [Crossref] [PubMed]
- Skeie GO, Aarli JA, Gilhus NE. Titin and ryanodine receptor antibodies in myasthenia gravis. Acta Neurol Scand Suppl 2006;183:19-23. [Crossref] [PubMed]
- Meriggioli MN, Sanders DB. Muscle autoantibodies in myasthenia gravis: beyond diagnosis? Expert Rev Clin Immunol 2012;8:427-38. [Crossref] [PubMed]
- Choi Decroos E, Hobson-Webb LD, Juel VC, et al. Do acetylcholine receptor and striated muscle antibodies predict the presence of thymoma in patients with myasthenia gravis? Muscle Nerve 2014;49:30-4. [Crossref] [PubMed]
- Suzuki S, Utsugisawa K, Nagane Y, et al. Three types of striational antibodies in myasthenia gravis. Autoimmune Dis 2011;2011:740583
- Suzuki S, Nishimoto T, Kohno M, et al. Clinical and immunological predictors of prognosis for Japanese patients with thymoma-associated myasthenia gravis. J Neuroimmunol 2013;258:61-6. [Crossref] [PubMed]
- Romi F, Suzuki S, Suzuki N, et al. Anti-voltage-gated potassium channel Kv1.4 antibodies in myasthenia gravis. J Neurol 2012;259:1312-6. [Crossref] [PubMed]
- Klein L, Hinterberger M, Wirnsberger G, et al. Antigen presentation in the thymus for positive selection and central tolerance induction. Nat Rev Immunol 2009;9:833-44. [Crossref] [PubMed]
- Criswell LA, Pfeiffer KA, Lum RF, et al. Analysis of families in the multiple autoimmune disease genetics consortium (MADGC) collection: the PTPN22 620W allele associates with multiple autoimmune phenotypes. Am J Hum Genet 2005;76:561-71. [Crossref] [PubMed]
- Ueda H, Howson JM, Esposito L, et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature 2003;423:506-11. [Crossref] [PubMed]
- Chuang WY, Strobel P, Gold R, et al. A CTLA4high genotype is associated with myasthenia gravis in thymoma patients. Ann Neurol 2005;58:644-8. [Crossref] [PubMed]
- Zheng K, Zhang J, Zhang P, et al. PTPN22 and CTLA-4 gene polymorphisms in resected thymomas and thymus for myasthenia gravis. Thorac Cancer 2012;3:307-12. [Crossref] [PubMed]
- Chuang WY, Strobel P, Belharazem D, et al. The PTPN22gain-of-function+1858T(+) genotypes correlate with low IL-2 expression in thymomas and predispose to myasthenia gravis. Genes Immun 2009;10:667-72. [Crossref] [PubMed]
- Belharazem D, Schalke B, Gold R, et al. cFLIP overexpression in T cells in thymoma-associated myasthenia gravis. Ann Clin Transl Neurol 2015;2:894-905. [Crossref] [PubMed]
- Rieker RJ, Schirmacher P, Schnabel PA, et al. Thymolipoma. A report of nine cases, with emphasis on its association with myasthenia gravis. Surg Today 2010;40:132-6. [Crossref] [PubMed]
- Huang CS, Li WY, Lee PC, et al. Analysis of outcomes following surgical treatment of thymolipomatous myasthenia gravis: comparison with thymomatous and non-thymomatous myasthenia gravis. Interact Cardiovasc Thorac Surg 2014;18:475-81. [Crossref] [PubMed]
- Schoser B, Eymard B, Datt J, et al. Lambert-Eaton myasthenic syndrome (LEMS): a rare autoimmune presynaptic disorder often associated with cancer. J Neurol 2017;264:1854-63. [Crossref] [PubMed]
- Nalbantoglu M, Kose L, Uzun N, et al. Lambert-Eaton myasthenic syndrome associated with thymic neuroendocrine carcinoma. Muscle Nerve 2015;51:936-8. [Crossref] [PubMed]
- Morimoto M, Osaki T, Nagara Y, et al. Thymoma with Lambert-Eaton myasthenic syndrome. Ann Thorac Surg 2010;89:2001-3. [Crossref] [PubMed]
- Shillito P, Molenaar PC, Vincent A, et al. Acquired neuromyotonia: evidence for autoantibodies directed against K+ channels of peripheral nerves. Ann Neurol 1995;38:714-22. [Crossref] [PubMed]
- Irani SR, Alexander S, Waters P, et al. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan's syndrome and acquired neuromyotonia. Brain 2010;133:2734-48. [Crossref] [PubMed]
- Lai M, Huijbers MG, Lancaster E, et al. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol 2010;9:776-85. [Crossref] [PubMed]
- Vincent A, Irani SR. Caspr2 antibodies in patients with thymomas. J Thorac Oncol 2010;5:S277-80. [Crossref] [PubMed]
- Torres-Vega E, Mancheno N, Cebrian-Silla A, et al. Netrin-1 receptor antibodies in thymoma-associated neuromyotonia with myasthenia gravis. Neurology 2017;88:1235-42. [Crossref] [PubMed]
- Isaacs H. A Syndrome of Continuous Muscle-Fibre Activity. J Neurol Neurosurg Psychiatry 1961;24:319-25. [Crossref] [PubMed]
- Gutmann L, Gutmann L. Myokymia and neuromyotonia 2004. J Neurol 2004;251:138-42. [Crossref] [PubMed]
- Fleisher J, Richie M, Price R, et al. Acquired neuromyotonia heralding recurrent thymoma in myasthenia gravis. JAMA Neurol 2013;70:1311-4. [PubMed]
- Skeie GO, Apostolski S, Evoli A, et al. Guidelines for treatment of autoimmune neuromuscular transmission disorders. Eur J Neurol 2010;17:893-902. [Crossref] [PubMed]
- Irani SR, Pettingill P, Kleopa KA, et al. Morvan syndrome: clinical and serological observations in 29 cases. Ann Neurol 2012;72:241-55. [Crossref] [PubMed]
- Iorio R, Lennon VA. Neural antigen-specific autoimmune disorders. Immunol Rev 2012;248:104-21. [Crossref] [PubMed]
- Gadoth A, Pittock SJ, Dubey D, et al. Expanded phenotypes and outcomes among 256 LGI1/CASPR2-IgG-positive patients. Ann Neurol 2017;82:79-92. [Crossref] [PubMed]
- Petit-Pedrol M, Armangue T, Peng X, et al. Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: a case series, characterisation of the antigen, and analysis of the effects of antibodies. Lancet Neurol 2014;13:276-86. [Crossref] [PubMed]
- Spatola M, Petit-Pedrol M, Simabukuro MM, et al. Investigations in GABAA receptor antibody-associated encephalitis. Neurology 2017;88:1012-20. [Crossref] [PubMed]
- Honnorat J, Cartalat-Carel S, Ricard D, et al. Onco-neural antibodies and tumour type determine survival and neurological symptoms in paraneoplastic neurological syndromes with Hu or CV2/CRMP5 antibodies. J Neurol Neurosurg Psychiatry 2009;80:412-6. [Crossref] [PubMed]
- Yu Z, Kryzer TJ, Griesmann GE, et al. CRMP-5 neuronal autoantibody: marker of lung cancer and thymoma-related autoimmunity. Ann Neurol 2001;49:146-54. [Crossref] [PubMed]
- Dell'Amore A, Asadi N, Caroli G, et al. Paraneoplastic dermatomyositis as presentation of thymic carcinoma. Gen Thorac Cardiovasc Surg 2013;61:422-5. [Crossref] [PubMed]
- Stefanou MI, Komorowski L, Kade S, et al. A case of late-onset, thymoma-associated myasthenia gravis with ryanodine receptor and titin antibodies and concomitant granulomatous myositis. BMC Neurol 2016;16:172. [Crossref] [PubMed]
- Kon T, Mori F, Tanji K, et al. Giant cell polymyositis and myocarditis associated with myasthenia gravis and thymoma. Neuropathology 2013;33:281-7. [Crossref] [PubMed]
- Graus F, Arino H, Dalmau J. Paraneoplastic neurological syndromes in Hodgkin and non-Hodgkin lymphomas. Blood 2014;123:3230-8. [Crossref] [PubMed]
- Briani C, Vitaliani R, Grisold W, et al. Spectrum of paraneoplastic disease associated with lymphoma. Neurology 2011;76:705-10. [Crossref] [PubMed]
- Iorio R, Sillevis Smitt P. Paraneoplastic Cerebellar Degeneration. In: Gruol DL, Koibuchi N, Manto M, et al., editor. Essentials of Cerebellum and Cerebellar Disorders. Springer, 2016.
- Bernal F, Shams'ili S, Rojas I, et al. Anti-Tr antibodies as markers of paraneoplastic cerebellar degeneration and Hodgkin's disease. Neurology 2003;60:230-4. [Crossref] [PubMed]
- de Graaff E, Maat P, Hulsenboom E, et al. Identification of delta/notch-like epidermal growth factor-related receptor as the Tr antigen in paraneoplastic cerebellar degeneration. Ann Neurol 2012;71:815-24. [Crossref] [PubMed]
- Iorio R, Damato V, Mirabella M, et al. Cerebellar degeneration associated with mGluR1 autoantibodies as a paraneoplastic manifestation of prostate adenocarcinoma. J Neuroimmunol 2013;263:155-8. [Crossref] [PubMed]
- Lopez-Chiriboga AS, Komorowski L, Kumpfel T, et al. Metabotropic glutamate receptor type 1 autoimmunity: Clinical features and treatment outcomes. Neurology 2016;86:1009-13. [Crossref] [PubMed]
- Marignier R, Chenevier F, Rogemond V, et al. Metabotropic glutamate receptor type 1 autoantibody-associated cerebellitis: a primary autoimmune disease? Arch Neurol 2010;67:627-30. [Crossref] [PubMed]
- Lancaster E, Martinez-Hernandez E, Titulaer MJ, et al. Antibodies to metabotropic glutamate receptor 5 in the Ophelia syndrome. Neurology 2011;77:1698-701. [Crossref] [PubMed]
- Rivera C, Arame A, Jougon J, et al. Prognostic factors in patients with primary mediastinal germ cell tumors, a surgical multicenter retrospective study. Interact Cardiovasc Thorac Surg 2010;11:585-9. [Crossref] [PubMed]
- Dalmau J, Graus F, Villarejo A, et al. Clinical analysis of anti-Ma2-associated encephalitis. Brain 2004;127:1831-44. [Crossref] [PubMed]
- Dalmau J, Lancaster E, Martinez-Hernandez E, et al. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol 2011;10:63-74. [Crossref] [PubMed]
- Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol 2013;12:157-65. [Crossref] [PubMed]
Cite this article as: Iorio R, Spagni G, Evoli A. Paraneoplastic neurological syndromes associated with mediastinal tumors. Mediastinum 2018;2:8.