
Primary germ cell tumors of the mediastinum: a review
Introduction
The presence of primary mediastinal germ cell tumors (M-GCT) has been recognized for over 100 years. However, due to the reluctance in the past to accept their occurrence in the mediastinal location, many tumors were diagnosed under different names while in reality represented M-GCT. Credit for the possible first description of a mediastinal teratoma is given to Gordon (1) who in 1827 reported a tumor in the mediastinal location in a descriptive manner—a tumor containing hair and teeth. If we were to recognize such description as the starting point, then it took more than 50 year to recognize that mediastinal seminoma also existed in the mediastinal location (2). Therefore, it has been only over the last 50 years that we have attempted to shed some light not only in terms of the spectrum of these tumors in the mediastinum but also in terms of clinical associations, incidence, classification, staging, and other details that are important in the assessment of these tumors. However, it is also important to highlight that these tumors are not common, and due to their rarity, only a few comprehensive series of these cases have been presented in the literature. One additional issue that is faced in today’s practice is that often the diagnosis of M-GCT is done with a mediastinoscopic biopsy, and once the diagnosis of GCT is established, it is likely that the patient receives medical treatment before the tumor is surgically removed, thus making histopathological appraisal of the tumor very difficult as often the tumor has become necrotic. This type of practice in essence has made histopathological classification much more difficult, if not impossible, as often the amount of necrosis in a given tumor far exceeds viable tumor. Because of that practice, previous large series of cases play an important role in guiding histopathological assessment and staging.
Also important to highlight is the continuous usage of the term mediastinal GCT, which although appropriate, perhaps does not convey that M-GCT are likely to be of thymic origin. Thus, the better nomenclature may be that of thymic-GCT (T-GCT), just the same as we use it for thymic carcinoma or other tumors in that region.
Incidence
The true incidence of M-GCT is difficult to estimate, as there are only a few large series of these tumors. In the past, the occurrence of M-GCT had been estimated to be somewhere between 1–20%, depending on the tumors reported (3-5). In a more complete review of mediastinal tumors in general, including adult and pediatric cases, Mullen and Richardson (6) identified 881 cases corresponding to 702 adults and 179 children. The authors documented an incidence of 15% of M-GCT in the adult population and 24% of M-GCT in the pediatric population. On the contrary, Davis et al. (7) estimated a 3% of M-GCT after reviewing 2,399 cases of mediastinal tumors in general, and identifying only 72 M-GCT over a period of 20 years. Perhaps one of the most difficult issues in estimating the incidence of M-GCT is determining what is to be included every tumor—benign or malignant? Only malignant tumors? However, in general practice, the majority of mediastinal tumors are dominated by lymphomas, thymomas, thymic carcinoma, and neuroendocrine carcinomas, leaving the M-GCT with a small proportion of cases.
Even though the true general incidence of M-GCT is difficult to estimate, the incidence of the different tumors that may be seen as primary M-GCT is somewhat easier to predict. In our experience with M-GCT in general, we documented 322 cases (8) in which we observed that teratomatous tumors followed by seminomas represent the bulk of M-GCT, leaving the presence of pure non-seminomatous/non-teratomatous tumors with a relative small percentage. One important feature that we have also noted is the predominance of males over females in the occurrence of these tumors in a proportion of approximately 9:1. As a matter of fact, in our experience, the times that we encountered M-GCT in female patients, the tumors have been invariably teratomatous tumors.
Classification
In 1997, based on our earlier experience with 322 cases, we proposed a classification (8), which as stated earlier is similar to that followed in other organ systems. However, there are a few semantic changes in order to avoid communication issues regarding other possible tumors in the mediastinum that may have similar names. One example of it will be the use of the term “mixed tumor”, which although descriptive, may be confused or misunderstood for a different tumor “mixed tumor”, which is of salivary gland origin, and that may occur also in the mediastinal compartment. Therefore, over the years, we have followed our classification:
- Teratoma
- Mature
- Immature
- With malignant component
- Type I—with another GCT [seminoma, yolk sac tumor (YST), embryonal carcinoma (EC), choriocarcinoma]
- Type II—with a malignant epithelial neoplasm (adenocarcinoma, squamous cell carcinoma, etc.)
- Type III—with a malignant mesenchymal component (rhabdomyosarcoma, angiosarcoma, etc.)
- Type IV—any combination of previous types
- Seminoma
- EC
- Choriocarcinoma
- Combined germ cell tumor
- A combination of any of the above tumors without teratomatous elements. If teratomatous elements are present, then the tumors should be classified as within the different types of teratomas.
In addition to this histopathological classification, it is also important to provide a percentage of the tumors present in those tumors in which the neoplasms have different components. The treating physician to determine specific treatment could use such information, and also it may be helpful in predicting clinical outcome.
Staging
As it would be expected, the proper staging should also follow the use of the histopathological classification. Even though, in the current practice many times that may not be possible, it is advisable that an attempt should be made to properly stage these tumors. The following staging system was also developed along with the histopathological classification:
- Stage I—tumor limited to the mediastinal compartment without invasion into adjacent organs such as pleura or pericardium
- Stage II—tumors show macro or microscopic invasion into adjacent structures such as pleura, pericardium, and great vessels
- Stage III—metastatic disease
- IIIA—metastasis in intrathoracic organs (lymph nodes, lung, etc.)
- IIIB—extra-thoracic metastasis.
Clinical features
The gamut of clinical symptoms or clinical associations that has been reported with M-GCT is extensive. Although a proportion of patients may present with no symptoms, others may present with non-specific symptoms such as cough, fever, chest pain, hemoptysis, dyspnea, pericarditis, and superior vena cava syndrome. On the other hand, M-GCT may also be associated with Klinefelter syndrome, insulin production in cases of teratomatous lesions, and hematologic malignancies (9-19). Although radiologically these tumors will appear as anterior mediastinal masses of variable size, some studies have identified the presence of calcifications in about 20% of cases of teratomas, and in some unusual cases, the presence of teeth and fat fluid levels will favor the interpretation of a teratomatous lesion (20).
Pathological features
Macroscopic features
Grossly, with the exception of teratomatous tumors, which are commonly cystic lesions, containing sebaceous material, hair, cartilage, or other elements, others M-GCT are much more difficult to distinguish from other mediastinal tumors. In general, M-GCT may present as either well-circumscribed tumors within the mediastinum, while other tumors may show ill-defined borders and more infiltrative pattern. Also the presence of hemorrhage and necrosis may vary in these tumors as some tumors like choriocarcinomas may show extensive areas of hemorrhage and necrosis while others may show a light brown homogeneous surface. In some tumors, cystic changes have been associated, namely with seminomas and YSTs.
Histological features
M-GCTs are distinct from one another and as such will be presented separately.
Teratomas: the hallmark of teratomatous tumors is the presence of tissues belonging to the three germinal layers. In cases of mature teratomas, the tumor at light microscopy will show the presence of different mature tissues, i.e., glial tissue, pancreas, muscle, and squamous epithelium, among others (Figure 1A). In cases of immature teratoma, the hallmark is the presence of immature neuroepithelium, arranged in the form of tubules or neural-rosettes (Figure 1B), which could be embedded in neutrophil. It is common to see in immature teratoma other mature elements. In cases of teratomas with another malignant component, it is essential to determine what the malignant component is, so that the proper classification may be stated. The presence of another malignant GCT such as seminoma, YST, etc. would classify the tumors as type I; while type II is associated with an epithelial malignancy such as squamous cell carcinoma, adenocarcinoma, etc.; type III, with a malignant mesenchymal tumor (Figure 1C), such as rhabdomyosarcoma, angiosarcoma, etc.; and type IV would be a mixture of any of the above histologies.
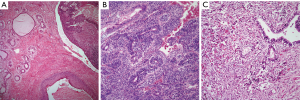
Immunohistochemical studies are not needed to make a diagnosis of teratoma. However, it is possible that in cases of type III teratomas, the use of immunohistochemistry may be of aid in identifying the specific malignant mesenchymal component (desmin, CD-34, CD31, etc.).
Seminoma: these tumors are characterized by the presence of sheets of neoplastic cells, which in some areas may have a subtle nested pattern separated by thin fibroconnective tissue with an inflammatory response composed mainly of lymphocytes. The neoplastic cells are of medium size, round to oval with eosinophilic cytoplasm, round to oval nuclei, and conspicuous nucleoli (Figure 2A,B). Seminomas may also show the presence of a non-caseous granulomatous reaction, remnants of thymic tissue, and lymphoid hyperplasia. Prominent cystic changes have been described in a small percentage of cases (21).
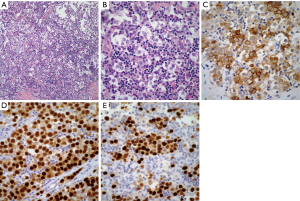
Histochemical stains although not as popular as they were in the past, continue providing good information regarding seminomas. The use of periodic acid-Schiff (PAS) to demonstrate the presence of glycogen in the neoplastic cells is an important tool in the diagnosis. Immunohistochemical studies are often used as an aid in the diagnosis of seminoma. The tumors are often positive for low-molecular weight cytokeratin CAM 5.2, PLAP, CD-117, SALL4, and OCT4 (22-25) (Figure 2C,D,E).
YST: the basic histopathological features of YST tumor are distinctive enough to make an unequivocal diagnosis on light microscopy. The tumor shows a reticular or microcystic growth pattern in which it is possible to see some gland-like structures or ductal-like areas. The tumor cells may be embedded in a fibrinous or myxoid stroma. Schiller-Duval bodies are a characteristic diagnostic feature of these tumors that allow for an easy interpretation if those structures are present. The neoplastic cellular proliferation is composed of small to medium size cells with round to oval nuclei and inconspicuous nucleoli. One other important diagnostic feature is the presence of intracytoplasmic hyaline globules (Figure 3A,B,C). Mitotic activity is minimal and marked cellular pleomorphism is rarely seen in these tumors. However, even though in the majority of cases the histology of the tumor is characteristic enough to make an unequivocal diagnosis, YST tumor is also known for showing different growth patterns that include hepatoid, sarcomatoid, and cystic (26-28). Therefore, it is essential to be familiar with those patterns. Nevertheless, it is also important to highlight that in the majority of cases with those unusual histological features, areas of more conventional YST are also present.
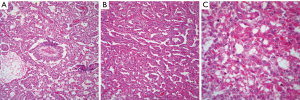
Immunohistochemical stains are also often used to confirm the diagnosis of YST, the tumor may show positive staining for alpha-fetoprotein (AFP), CEA, keratin, alpha-1-antitripsin, vimentin, PLAP, EMA, NSE, and SALL4 (29,30). In daily practice the immunostain more often use is AFP.
EC: this tumor in its pure form is unusual and it is often accompanied by another GCT namely YST. However, the histopathological features of this tumor are also characteristic enough to make an unequivocal diagnosis on light microscopy. The tumor characteristically shows the presence of a glandular growth pattern in which the neoplastic cells are large, with round nuclei, and prominent nucleoli (Figure 4A,B). Mitosis and cellular atypia are commonly identified and the tumor cells may be embedded in a necrotic background.
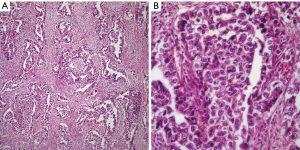
Immunohistochemical stains also play an important role in confirming the diagnosis of EC. These tumors similar to YST are positive for AFP, EMA, and keratin. However, one important immunostain that is important to keep in mind is CD30, which is a common stain used in the diagnosis of anaplastic large cell lymphoma (ALCL). CD30 is also positive in EC, which in the context of an EMA positive tumor in a young patient will also alert about the possibility of ALCL. In this context and if in doubt, the use of ALK-1 should be performed as EC is generally negative for ALK-1, and a wider panel of immunohistochemical stains may prove useful.
Choriocarcinoma: it represents the least common tumor in the mediastinum and it has been reported only rarely in the literature and often as case reports (31-37). The largest series of these tumors was presented more recently and it included only eight well-documented cases of anterior mediastinal choriocarcinomas (38). The hallmark of this tumor is the presence of cytotrophoblastic and syncytiotrophoblastic cells. The former are large cells with round nuclei, conspicuous nucleoli, and clear cytoplasm, while the latter cells are multinucleated cells with abundant eosinophilic cytoplasm. The tumor characteristically shows extensive areas of hemorrhage and/or necrosis. Cellular atypia and mitotic activity are easily identified (Figure 5A,B).
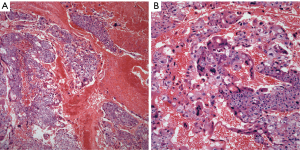
The most common immunohistochemical stain used in the diagnosis of choriocarcinoma is human chorionic gonadotropin (HCG). However, other stains including keratin, PLAP, EMA, and AFP may show positive staining in tumor cells.
Combined GCT: according to our classification schema, these tumors should show at least two components of a GCT, however, none of those components should be a teratoma. Therefore, one can see seminoma and YST or YST with EC. Essentially any combination of the non-teratomatous lesions will fit into this category. The use of Immunohistochemical stains will depend on the type of tumor present.
Discussion
Primary M-GCT represents a small percentage of primary mediastinal tumors when compared to the total occurrence of mediastinal pathology. However, they do represent a special subtype of neoplasms that require special attention in order to arrive at a specific diagnosis, so that patients may be treated accordingly. Prior to 1997, most of what we knew about M-GCT was essentially either by anecdotal case reports or small series of cases. However, the report of 322 cases of primary M-GCT defined many of the clinical, radiological, and pathological features, which included the histopathology, staging and classification of these tumors (8,39,40). Due to the similar histopathological features that gonadal and mediastinal GCT share, it is always prudent to determine whether the tumor is not a metastatic disease from the gonads. However, on this particular point, it is also important to mention that such possibility has been studied in the past. For instance, Luna and Valenzuela-Tamaris (41) reported an autopsy study of 20 patients with M-GCT in whom the authors paid special attention to the possibility of an occult testicular tumor. The testes were sectioned completely and only in two cases the authors identified a small occult tumor or a well-defined scar. When compared those cases to the M-GCT, there was no correlation as the M-GCT were of the non-seminomatous type. In a different study on testicular GCT, Johnson et al. (42) reported 78 autopsies in patients with testicular GCT with no metastatic disease to the mediastinum. Lynch and Blewitt (43) in a description of mediastinal choriocarcinoma cited a study by Houghton of 220 testicular GCT in which the authors did not identified any metastatic disease to the mediastinum. Therefore, even though such occurrences have been reported, the possibilities are high for a primary M-GCT in the setting of a young patient with a bulky anterior mediastinal mass. Once the tumor has been established to be primary in the mediastinum, then it is highly important to document the type of tumor that it may be. As stated before, in today’s practice such luxury may not be possible as most cases in which a mediastinoscopic biopsy is diagnosed as M-GCT, likely the patient will undergo medical treatment before any surgical attempt is made. Thus often the original biopsy is the only determinant about the possible M-GCT that may be present.
In terms of prognosis, based on our experience, those cases in which the tumor is of the mature type of teratoma, the followed up after surgical resection is accomplished is rather good as those patients will likely be cured. In cases in which the diagnosis is that of an immature teratoma, the clinical outcome of those patients will be determined not only by the extent of the immature component 5%, 50%, or 90% but also it may be related to the age of the patient. It has been stated in the past that younger patients (children) have better clinical outcome than adult patients. On the other hand, the more complex issue is for teratomas with malignant component. In this setting, the presence of a mesenchymal component (sarcoma) is often associated with poor outcome. For pure seminomas, the clinical outcome is related to the extent of disease—limited to the mediastinum or involving other structures—essentially staging of the tumor at the time of diagnosis. It is known that seminomas are radiosensitive tumors and respond well to treatment. However, in some series of cases, more aggressive disease has been documented. In our experience with 120 cases (39), we documented metastatic disease to the lung, lymph node, liver, and bone, thus showing that these tumors may follow an aggressive behavior. However, the number of patients in which the tumors behave more aggressively was a minority in comparison to those who did well. More recently, a different study has shown 100% survival at 5 years (44). In our experience with 65 patients in whom a follow up was obtained (mean: 10 years), we were able to identified only 16 deaths. The clinical follow-up in cases of non-seminomatous M-GCT may be different and will depend on the tumor in question. For instance, in cases of YST, even though more modern treatment options have improved the survival in these patients, recurrences and deaths are commonly seen (45). However, different series of non-seminomatous GCT have provided different percentages that vary from 10% to 50% for the 5-year survival rate (46,47). In our experience with non-seminomatous tumors and in particular with YST, we encountered that only 13 patients survived 2 years. However, it is also important to highlight that when the study took place, current more modern treatment protocols may not have been available. It is possible that the recurrence rate of EC is higher than that seen in YST, however, in terms of clinical outcome, both tumors may show similar outcome. In addition, it is important to highlight that YST and EC often occur as a combine tumor. On the other hand, the clinical outcome for choriocarcinomas is rather poor. One important determinant factor is that by the time the tumor is diagnosed, it is already in late stages as the patient may present with more extensive thoracic disease or disease below the diaphragm, making the clinical outcome more complicated.
From the practical point of view regarding the differential diagnosis in M-GCT, it can be rather limited or extensive based on the tumor in question. In cases of teratomatous lesions, the diagnosis is rather easy; however, the most important factor is to determine the type of teratomatous lesion, i.e., immature or with malignant component. In the cases of seminoma, the diagnosis can be straightforward; however, if the tumor has a granulomatous reaction and cystic changes, other possibilities may be included. In this setting, one needs to consider the possibility of Hodgkin lymphoma, and the proper immunohistochemical stains should be performed. For cases of EC, the most important differential diagnosis would be with ALCL lymphoma as both tumors may share EMA and CD30 as positive immunohistochemical markers. In cases of choriocarcinoma, the possibility of a pleomorphic carcinoma needs to be considered. However, in this setting the presence of a bulky anterior mediastinal mass in a young patient would be more in keeping with a primary mediastinal tumor.
In short, M-GCT represent an important group of tumors that one needs to keep in mind as proper diagnosis is highly important in the clinical outcome and treatment. Ideally, it is advisable to determine the type and staging of these tumors. However, current oncological practice may preclude from such luxury. Therefore, it is essential that the mediastinoscopic biopsy be properly interpreted, as it will be the one that will determine treatment options for these patients. The use of immunohistochemical stains is advised in cases in which the histology may not be the typical one. Clinical outcome in these patients will depend largely on the type of tumor and the stage at the time of diagnosis. Current treatment options have probably improved the survival in these patients.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor Mirella Marino for the series “Diagnostic Problems in Anterior Mediastinum Lesions” published in Mediastinum. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med.2017.12.01). The series “Diagnostic Problems in Anterior Mediastinum Lesions” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gordon JA. Case of Tumor in the Anterior Mediastinum, containing Bone and Teeth. Med Chir Trans 1827;13:12-6. [Crossref] [PubMed]
- Woolner LB, Jamplis RW, Kirklin JW. Seminoma (germinoma) of the anterior mediastinum. New Engl J Med 1955;252:653-7. [Crossref] [PubMed]
- Hainsworth JD, Greco FA. Extragonadal germ cell tumors and unrecognized germ cell tumors. Seminars in Oncology 1992;19:119-27. [PubMed]
- Dulmet EM, Macchiarini P, Suc B, et al. Germ cell tumors of the mediastinum. Cancer 1993;72:1894-901. [Crossref] [PubMed]
- Dehner LP. Germ cell tumors of the mediastinum. Semin Diagn Pathol 1990;7:266-84. [PubMed]
- Mullen B, Richardson DJ. Primary anterior mediastinal tumors in children and adults. Ann Thorac Surg 1986;42:338-45. [Crossref] [PubMed]
- Davis RD, Oldham NH, Sabiston DC. Primary cysts and neoplasms of the mediastinum: Recent changes in clinical presentation, methods of diagnosis. management, and results. Ann Thorac Surg 1987;44:229-37. [Crossref] [PubMed]
- Moran CA, Suster S. Primary germ cell tumors of the mediastinum: I. Analysis of 322 cases with special emphasis on teratomatous lesions and a proposal for histopathologic classification and clinical staging. Cancer 1997;80:681-90. [Crossref] [PubMed]
- Lachman MF, Kim K, Koo BC. Mediastinal teratoma associated with klinefelter's syndrome. Arch Pathol Lab Med 1986;110:1067-71. [PubMed]
- Aravanis C, Papasteriades E, Steriotis J. Recurrent pericarditis due to cystic teratoma of the mediastinum. Angiology 1980;31:427-30. [Crossref] [PubMed]
- Robertson JM, Fee HJ, Mulder DG. Mediastinal teratoma causing life-threatening hemoptysis. Am J Dis Child 1981;135:148-50. [Crossref] [PubMed]
- Deenadayalu RP, Tuuri D, Dewall RA, et al. Intrapericardial teratoma and bronchogenic cyst. J Thorac Cardiovasc Surg 1974;67:945-52. [PubMed]
- Honicky RE, dePapp EW. Mediastinal teratoma with endocrine function. Am J Dis Child 1973;126:650-3. [PubMed]
- Sogge MR, McDonald SD, Cofold PB. The malignant potential of the dysgenetic germ cell in klinefelter's syndrome. Am J Med 1979;66:515. [Crossref] [PubMed]
- Nichols CR, Heerema NA, Palmer C, et al. Klinefelter's syndrome associated with mediastinal germ cell neoplasm. Journal of Clinical Oncology 1987;5:1290-4. [Crossref] [PubMed]
- Floret D, Renaud H, Monnet P. Sexual precocity and thoracic polyembryoma: Klinefelter syndrome? J Pediatr 1979;94:163. [Crossref] [PubMed]
- Garnick MB, Griffin JD. Idiopathic thrombocytopenia in association with extragonadal germ cell cancer. Ann Intern Med 1983;98:926-7. [Crossref] [PubMed]
- Nichols CR, Roth BJ, Heerema N, et al. Hematologic neoplasia associated with primary medistinal germ cell tumors. N Engl J Med 1990;322:1425-9. [Crossref] [PubMed]
- Orazi A, Neiman RS, Ulbright TM, et al. Hematopoietic precursor cells within the yolk sac tumor component are the source of secondary hematopoietic malignancies in patients with medistinal germ cell tumors. Cancer 1993;71:3873-81. [Crossref] [PubMed]
- Rosado-de-Christenson ML, Templeton P, et al. Mediastinal germ cell tumors: Radiologic and Pathologic correlation. Radiographics 1992;12:1013-30. [Crossref] [PubMed]
- Moran CA, Suster S. Mediastinal seminomas with prominent cystic changes: a clinicopathologic study of 10 cases. Am J Surg Pathol 1995;19:1047-53. [Crossref] [PubMed]
- Suster S, Moran CA, Dominguez-Malagon H, et al. Germ cell tumors of the mediastinum and testis: a comparative immunohistochemical study of 120 cases. Human Pathology 1998;29:737-42. [Crossref] [PubMed]
- Weissferdt A, Moran CA. Primary mediastinal "thymic" seminomas. Adv Anat Pathol 2012;19:75-80. [Crossref] [PubMed]
- Jung SM, Chu PH, Shiu TF, et al. Expression of OCT4 in primary germ cell tumors and thymoma in the mediastinum. Appl Immunohistochem Mol Morphol 2006;14:273-5. [Crossref] [PubMed]
- Liu A, Cheng L, Du J, et al. Diagnostic utility of novel stem cell markers SALL4, OCT4, NANOG, SOX2, UTF1, and TCL1 in primary mediastinal germ cell tumors. Am J Surg Pathol 2010;34:697-706. [PubMed]
- Moran CA, Suster S. Yolk sac tumors of the mediastinum with prominent spindle cell features. Am J Surg Pathol 1997;21:1173-7. [Crossref] [PubMed]
- Moran CA, Suster S. Mediastinal yolk sac tumors associated with prominent multilocular cystic changes of thymic epithelium: a clinicopathologic and immunohistochemical study of five cases. Modern Pathology 1997;10:800-3. [PubMed]
- Moran CA, Suster S. Primary yolk sac tumors of the mediastinum prominent hepatoid features: a clinicopathologic and immunohistochemical study of 4 cases. Am J Surg Pathol 1997;21:1210-4. [Crossref] [PubMed]
- Truong LD, Harris L, Mattioli C, et al. Endodermal sinus tumor of the mediatinum. Cancer 1986;58:730-9. [Crossref] [PubMed]
- Kurman RJ, Scardino PT, McIntire KR, et al. Cellular localization of alpha-fetoprotein and human chorionic gonadotrophin in germ cell tumors of the testis using an indirect immunoperoxidase technique. Cancer 1977;40:2136-51. [Crossref] [PubMed]
- Sickles EA, Belliveau RE, Wiernik PH. Primary mediatinal choriocarcinoma in the male. Cancer 1974;33:1196-203. [Crossref] [PubMed]
- Greenwood SM, Goodman JR, Schneider G, et al. Choriocarcinoma in a man. Am J Med 1971;51:416-22. [Crossref] [PubMed]
- Storm PB, Fallon B, Bunge RG. Mediastinal choriocarcinoma in a chromatin-positive boy. J Urol 1976;116:838-40. [Crossref] [PubMed]
- Hsueh YS, Tsung SH, Shamsai R, et al. Primary mediastinal choriocarcinoma in a man with an abnormal chromosome. South Med J 1984;77:1466-9. [Crossref] [PubMed]
- Kathuria S, Jablokov VR. Primary choriocarcinoma of mediastinum with immunohistochemical study and review of the literature. J Surg Oncol 1987;34:39-42. [Crossref] [PubMed]
- Connolly CE, Gillan J, Maguire R, et al. Primary choriocarcinoma of the mediastinum. Ir J Med Sci 1979;148:20-2. [Crossref] [PubMed]
- Candes FP, Ajinkya MS. Primary mediastinal choriocarcinoma (case report). J Postgrad Med 1987;33:219-21. [PubMed]
- Moran CA, Suster S. Primary mediastinal choriocarcinomas: A clinicopathologic and immunohistochemical study of eight cases. Am J Surg Pathol 1997;21:1007-12. [Crossref] [PubMed]
- Moran CA, Suster S, Przygodski RM, et al. Primary germ cell tumors of the mediastinum. II. Mediastinal seminomas - a clinicopathologic and immunohistochemical study of 120 cases. Cancer 1997;80:691-98. [Crossref] [PubMed]
- Moran CA, Suster S, Koss MN. Primary germ cell tumors of the mediastinum. III Yolk sac tumor, embryonal carcinoma, choriocarcinoma, and combined nonteratomatous germ cell tumors of the mediastinum - A clinicopathologic and immunohistochemical study of 64 cases. Cancer 1997;80:699-707. [Crossref] [PubMed]
- Luna MA, Valenzuela-Tamaris J. Germ cell tumors of the mediastinum, postmortem findings. Am J Clin Pathol 1976;65:450-4. [Crossref] [PubMed]
- Johnson DE, Appelt G, Samuels ML, et al. Metastases from testicular carcinoma: Study of 78 autopsied cases. Urology 1976;8:234-9. [Crossref] [PubMed]
- Lynch MJ, Blewitt GL. Choriocarcinoma arising in the male mediastinum. Thorax 1953;8:157-61. [Crossref] [PubMed]
- Biester RJ, Lippert MC, Mills SE. Late recurrence of a seminoma. J Urol 1987;137:749-50. [Crossref] [PubMed]
- Kurman RJ, Norris HJ. Endodermal sinus tumors of the ovary. Cancer 1976;38:2404-19. [Crossref] [PubMed]
- Nichols CR, Saxman S, Williams SD, et al. Primary mediastinal nonseminomatous germ cell tumors. Cancer 1990;65:1641-6. [Crossref] [PubMed]
- Kersh CR, Eisert DR, Constable WC. Primary malignant mediastinal germ-cell tumors and the contribution of radiotherapy: A southeastern multi-institutional study. Am J Clin Oncol 1987;10:302-6. [Crossref] [PubMed]
Cite this article as: Kalhor N, Moran CA. Primary germ cell tumors of the mediastinum: a review. Mediastinum 2018;2:4.


