
Single missense mutation as a signal of indolent thymic epithelial tumors: General Transcription Factor II-I (GTF2I)
Thymic epithelial tumors (TETs) are enigmatic malignancies consisting of diverse components of lymphocytes and epithelial cells (1) and we are only beginning to understand the molecular determinants of these tumors (2-8). Petrini and colleagues (2) first reported the identification of a missense mutation in general transcription factor II-I (GTF2I) (p.Leu424His in change to codon chromosome 7 c.74146970T>A) that highly occurred in type A thymomas demonstrating favorable survival (2). GTF2I β and δ isoforms are expressed in TETs, and in vitro experiments showed that both mutation isoforms could stimulate cell proliferation. In addition, thymic carcinomas have been found to possess a higher number of mutations than thymomas, with recurrent genomic alterations of well-known cancer genes such as TP53, CDKN2A, and BAP1 (2). Recently, we reported a clinically relevant molecular subtyping system for TETs, based upon distinct patterns of genomic alterations across several independent patient cohorts (8). Molecular classification of TETs was investigated in 120 patients from the cancer genome atlas (TCGA) using a multidimensional approach integrating analyses of DNA mutation, transcriptome expression, and somatic copy number alterations (SCNA), and 4 molecular subtypes were identified. In this TCGA cohort, a missense mutation in GTF2I (GTF2I group) was the most commonly identified gene mutation, present in 38% of TET patients. The next subtype was classified by unsupervised transcriptome clustering of GTF2I wild type tumors and represented TETs with abundant expression of genes associated with T cell signaling (TS group; 33%). Based upon SCNA analyses, the remaining two groups were categorized by their degree of chromosomal stability (CS group; 8%) or instability (CIN group; 21%) (Figure 1A). The clinical relevance of these molecular subtypes was demonstrated in the TCGA cohort: (I) the GTF2I mutation group was frequent in WHO class A and AB TETs and was correlated with a lower incidence of myasthenia gravis (MG); (II) the TS group occurred mainly in B1 and B2 WHO classifications; (III) the CIN group was prevalent in B3 and thymic carcinoma histology; (IV) CS and CIN groups were associated with an increased prevalence of MG (40%). Furthermore, overall survival (OS) and disease-free survival (DFS) were each favorable in the GTF2I group and unfavorable in the CIN group.
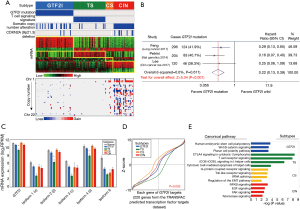
In this issue of Mediastinum, Feng and colleagues (9) also reported that the presence of the GTF2I mutation correlated with better prognosis in TETs (90.0% compared to 72.0% 5-year survival, and 86% compared to 56% 10-year survival, respectively; P=0.001). Further, GTF2I mutational status was an independent prognostic factor in multivariable analysis in 296 TET patients [hazard ratio (HR), 0.42; 95% confidential interval (CI), 0.18–0.98; P=0.045)]. Similar to previous reports (2,8), this group demonstrated that the GTF2I mutation was most prevalent in type A thymoma (87.0%), a relatively indolent TET, and the frequency of this mutation decreased with the degree of histological aggressiveness, with the lowest rate being observed in thymic carcinoma (7.7%). A meta-analysis with three studies (2,8,9) nicely demonstrates that TET patients with GTF2I mutation have better prognosis than those without GTF2I mutation (Figure 1B).
Transcription factor II-I (TFII-I) is a multifunctional protein that plays a role in the transcriptional control of several genes that regulate cell proliferation and developmental processes, and which is activated by the binding of TFII-I to the FOS promoter (10). Although the induction of GTF2I mutation in mouse fibroblast cell lines promoted cell proliferation compared with mock-transfected cells and their wild-type counterparts (2), and the loss of a region on chromosome 7 that contains the GTF2I locus has been associated with Williams-Beuren syndrome (11), very little is known about the function of GTF2I in human malignancy. According to mRNA data obtained from TCGA TETs, GTF2I mutation group showed similar mRNA expression of GTF2I and its isoforms to that of adjacent normal tissue, and of TETs within the CS and CIN groups (Figure 1C). Using known transcription factor binding site motifs within the TRANSFAC®, a database on transcription factors and their DNA binding sites (12), we calculated GTF2I activity for the 220 target mRNAs of the GTF2I transcription factor analyzed this for each molecular subtype. These data demonstrated that a GTF2I mutation may not influence the function of GTF2I mRNA as a transcription factor (Figure 1D). However, unsupervised clustering of mRNA transcriptome sequencing data of TETs demonstrated that all patients with GTF2I mutation were distinctly distributed into one specific cluster, implying that the GTF2I mutation may be a crucial driving mutation in TETs although the association between GTF2I mutation and the alteration of targeted transcription mRNAs has not been shown. Additionally, canonical pathway analyses were performed to investigate in further detail the biological properties associated with molecular subtypes. Ingenuity® Pathway Analysis (IPA) of differential mRNA expression showed that the GTF2I group was enriched for genes related to human embryonic stem cell pluripotency and Wnt/β-catenin signaling, which are involved in self-renewal of cells (Figure 1E). Comparison of protein expression among molecular subgroups revealed consistent findings that the GTF2I group demonstrated high expression of β-catenin, PIK3CA, and YAP1 proteins that are important in the stem cell pathway (8). The functional role and the underlying mechanism of GTF2I mutation in TETs requires further investigation.
With the advancement of targeted therapeutics, the molecular classification of TETs may become increasingly important. Whereas surgical resection is the mainstay of the treatment for most patients with TETs, effective systemic therapy is critical for patients with for advanced tumors requiring systemic therapy prior to surgery, unresectable tumors, and metastatic tumors, the latter which presently have only 19% 10-year OS (13). It is reasonable to hypothesize that the extent of surgical resection of TETs may one day be informed by a molecular classification system. For example, we may learn that GTF2I mutant TETs may be best served by minimally invasive thymectomy alone, and that a CIN subgroup TET of similar size should undergo extended thymectomy with mediastinal lymphadenectomy, and potentially adjuvant therapy. Similar considerations might be made for implementation or non-implementation of adjuvant therapy following surgical resection of any TETs depending upon the GTF2I mutational status.
The role of GTF2I mutation in advanced TETs deserves mention. In Feng’s study, 7.7% of thymic carcinomas, 10% of B3 thymomas, and 20% of B2 thymomas had a GTF2I mutation. Similarly, in Petrini’s study, 8% of thymic carcinomas, 21% of B3 thymomas, and 22% of B2 thymomas had this mutation. In our study, among 54 patients with advanced WHO classification (B2, B3, and thymic carcinoma), 13% had a GTF2I mutation and none of these patients suffered from recurrence in the study period. Similarly, among 21 patients with advanced Masaoka stage, the tumors of 5 patients (24%) demonstrated GTF2I mutation and these patients also did not experience recurrence. Thus, whereas the GTF2I mutation is less frequent in advanced tumors, it may have an important role as a biomarker in advanced disease.
While development of targeted therapy for thymoma and thymic carcinoma is currently in its infancy, experience with immunotherapy for advanced TETs is rapidly accumulating. A promising report of a phase II study of pembrolizumab [anti-Programmed Cell Death 1 (PD-1) antibody] for patients with recurrent thymic carcinoma revealed objective response [complete response (CR) + partial response (PR)] of 22.5% (9/40) and disease control [CR + PR + stable disease (SD)] of 72.5% (14). One patient had CR, 8 PR, 20 SD, and 11 had progression. Although six patients (15%) developed multiple grade 3–4 immune related adverse events, there were no treatment related deaths. A thorough understanding of the genomic characteristics of TETs may potentially facilitate development of a reliable biomarker to identify TET patients most likely to respond to checkpoint blockade and minimize toxicity by avoiding therapy in patients not poised to respond. Accordingly, integrative analysis of immunogenic determinants provides several potential clues that TET with GTF2I mutation may respond unfavorably to immune checkpoint inhibitors.
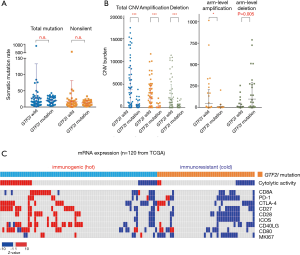
Firstly, the Wnt/β-catenin signaling pathway that is associated with the GTF2I mutation is known to correlate with cancer immune evasion and resistance to immunotherapy. For example, molecular analyses of metastatic human melanoma specimens showed correlation between up-regulation of the Wnt/β-catenin signaling and the absence of a T-cell gene expression signature which correlated with response to immune checkpoint inhibitors. These findings were corroborated in an autochthonous mouse melanoma models in which tumor-intrinsic active β-catenin signaling resulted in T-cell exclusion and resistance to anti-Cytotoxic T-Lymphocyte Associated protein 4 (CTLA-4)/anti-Programmed Death Ligand 1 (PD-L1) monoclonal antibody therapy (15). Secondly, Giaccone demonstrated that response to pembrolizumab in 15 patients with advanced TETs was not correlated with mutational burden (14), and mutational burden is not found to be different between GTF2I mutant and GTF2I wild type TETs within the TCGA cohort (Figure 2A). Analyses of copy number alternations (CNAs) in melanoma tumors identified a higher burden of copy number loss in non-responders to CTLA-4 and PD-1 blockade (16,17). If these findings can be extended to TETs, in which arm-level copy number loss was significantly higher in GTF2I mutant TETs (Figure 2B), GTF2I mutant TETs may not be expected to respond to checkpoint blockade. Finally, tumors in the GTF2I wild type group are enriched for genes related to immunostimulatory and immunoregulatory signaling such as cytolytic activity, PD-1, and CTLA-4 (Figure 2C). Such tumors may be poised to respond favorably to immune checkpoint blockade, and these data show good agreement with reports of PD-1 positivity (46%) and PD-L1 (23–80%) on TETs (18-20). Taken together, we can hypothesize that GTF2I wild type TETs are more immunogenic than GTF2I mutant TETs.
In summary, these data support a GTF2I mutation as the most frequent mutation in TETs, and the presence of this mutation as a correlate of favorable outcome. Further, as immunotherapy becomes an integral part of cancer care, GTF2I mutation may play a predictive role in resistance to immune checkpoint inhibitors for advanced or recurrent TETs. Deeper investigation into the molecular mechanisms underlying TETs and the TET molecular stratification framework could expedite the clinical application of GTF2I mutational status as an adjunct to clinical staging and can support the development of reasonable treatment options for TET patients.
Acknowledgments
The results shown here are in part based upon data generated by the TCGA Research Network:
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by Section Editor Zhuoqi Jia (Thoracic Department, the First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med.2017.11.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Engels EA. Epidemiology of thymoma and associated malignancies. J Thorac Oncol 2010;5:S260-5. [Crossref] [PubMed]
- Petrini I, Meltzer PS, Kim IK, et al. A specific missense mutation in GTF2I occurs at high frequency in thymic epithelial tumors. Nat Genet 2014;46:844-9. [Crossref] [PubMed]
- Okumura M, Fujii Y, Shiono H, et al. Immunological function of thymoma and pathogenesis of paraneoplastic myasthenia gravis. Gen Thorac Cardiovasc Surg 2008;56:143-50. [Crossref] [PubMed]
- Petrini I, Wang Y, Zucali PA, et al. Copy number aberrations of genes regulating normal thymus development in thymic epithelial tumors. Clin Cancer Res 2013;19:1960-71. [Crossref] [PubMed]
- Inoue M, Starostik P, Zettl A, et al. Correlating genetic aberrations with World Health Organization-defined histology and stage across the spectrum of thymomas. Cancer Res 2003;63:3708-15. [PubMed]
- Wang Y, Thomas A, Lau C, et al. Mutations of epigenetic regulatory genes are common in thymic carcinomas. Sci Rep 2014;4:7336. [Crossref] [PubMed]
- Girard N, Shen R, Guo T, et al. Comprehensive genomic analysis reveals clinically relevant molecular distinctions between thymic carcinomas and thymomas. Clin Cancer Res 2009;15:6790-9. [Crossref] [PubMed]
- Lee HS, Jang HJ, Shah R, et al. Genomic Analysis of Thymic Epithelial Tumors Identifies Novel Subtypes Associated with Distinct Clinical Features. Clin Cancer Res 2017;23:4855-64. [Crossref] [PubMed]
- Feng Y, Lei Y, Wu X, et al. GTF2I mutation frequently occurs in more indolent thymic epithelial tumors and predicts better prognosis. Lung Cancer 2017;110:48-52. [Crossref] [PubMed]
- Grueneberg DA, Henry RW, Brauer A, et al. A multifunctional DNA-binding protein that promotes the formation of serum response factor/homeodomain complexes: identity to TFII-I. Genes Dev 1997;11:2482-93. [Crossref] [PubMed]
- Enkhmandakh B, Makeyev AV, Erdenechimeg L, et al. Essential functions of the Williams-Beuren syndrome-associated TFII-I genes in embryonic development. Proc Natl Acad Sci U S A 2009;106:181-6. [Crossref] [PubMed]
- Rouillard AD, Gundersen GW, Fernandez NF, et al. The harmonizome: a collection of processed datasets gathered to serve and mine knowledge about genes and proteins. Database (Oxford) 2016;2016. pii: baw100.
- Hamaji M, Burt BM. Long-Term Outcomes of Surgical and Nonsurgical Management of Stage IV Thymoma: A Population-Based Analysis of 282 Patients. Semin Thorac Cardiovasc Surg 2015;27:1-3. [Crossref] [PubMed]
- Mccutcheon JN, Giaccone G, Thompson J, et al. Pembrolizumab in patients with recurrent thymic carcinoma: Results of a phase II study. American Society of Clinical Oncology 2017;35:8573.
- Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature 2015;523:231-5. [Crossref] [PubMed]
- Roh W, Chen PL, Reuben A, et al. Integrated molecular analysis of tumor biopsies on sequential CTLA-4 and PD-1 blockade reveals markers of response and resistance. Sci Transl Med 2017;9:eaan3788 [Crossref] [PubMed]
- Davoli T, Uno H, Wooten EC, et al. Tumor aneuploidy correlates with markers of immune evasion and with reduced response to immunotherapy. Science 2017;355:eaaf8399 [Crossref] [PubMed]
- Padda SK, Riess JW, Schwartz EJ, et al. Diffuse high intensity PD-L1 staining in thymic epithelial tumors. J Thorac Oncol 2015;10:500-8. [Crossref] [PubMed]
- Yokoyama S, Miyoshi H, Nakashima K, et al. Prognostic Value of Programmed Death Ligand 1 and Programmed Death 1 Expression in Thymic Carcinoma. Clin Cancer Res 2016;22:4727-34. [Crossref] [PubMed]
- Katsuya Y, Horinouchi H, Asao T, et al. Expression of programmed death 1 (PD-1) and its ligand (PD-L1) in thymic epithelial tumors: Impact on treatment efficacy and alteration in expression after chemotherapy. Lung Cancer 2016;99:4-10. [Crossref] [PubMed]
Cite this article as: Lee HS, Truong CY, Jang HJ, Burt BM. Single missense mutation as a signal of indolent thymic epithelial tumors: General Transcription Factor II-I (GTF2I). Mediastinum 2017;1:20.


