
Balance between less invasiveness and radical capability with safety
Various types of minimally invasive procedures have been developed for performing a thymectomy and resection of an anterior mediastinal tumor in order to avoid a sternotomy. Those are usually performed with either thoracic or infrasternal (subxiphoid) access. Recently, surgeons have been attempting to reduce the number and size of the access ports with the goal of less injury to the chest wall. A uniportal (single-port) approach is the final state of such so-called reduced port surgery.
Another recent topic of discussion in regard to minimally invasive thoracic surgery is non-intubated awake anesthesia. Non-intubated thoracic surgery (NITS) can be achieved through a local, usually epidural, anesthesia method in mildly sedated and spontaneously ventilating patients. One advantage is avoidance of traumatic side-effects caused by intubation under general anesthesia, thus making recovery easier and optimizing the clinical outcome.
Pompeo E, Mineo TC and colleagues began to apply thoracic epidural anesthesia (TEA) for NITS in the early era of thoracoscopic surgery and have reported several important findings thus far (1). Based on their results, they have evolutionally combined those two methods for patients with a thymoma associated with myasthenia gravis (MG) to successfully achieve ultra-minimally invasive thoracic surgery (2). I read with interest their study and comment here with deep respect for their work.
NITS for MG
Although general anesthesia provides surgeons a stable operative situation, intubation and use of paralytic agents can induce some adverse effects, such as airway trauma and arousal delay. Particularly in myasthenic patients, special attention in regard to the type of anesthesia and use of muscle relaxants is required (1). Tsunezuka et al. reported performance of an awake transsternal extended thymectomy in three low-risk MG patients under maintenance with TEA, which was employed to resolve the disadvantages of general anesthesia (3). They concluded that TEA without intubation is apparently favorable, in that muscle relaxants are not necessary, and laryngeal injury and potential postoperative respiratory failure can be avoided. Pompeo E and colleagues have also performed their uniportal thymectomy procedure with a similar anesthesia strategy and shown the advantages of TEA (2).
However, some grave problems can develop in such cases. First, if positive pressure ventilation is required because of an unexpected critical event, I wonder whether a laryngeal mask can maintain stable single-lung ventilation. Although they noted that opening of the left (contralateral) mediastinal pleura was not intentionally performed, both sides of the pleura can be easily injured during an extended thymectomy, for which wide dissection in the anterior mediastinum is required. Thus, the merits of an awake endoscopic thymectomy with TEA may reside more in relation to the infrasternal approach, in which opening of the pleura is not generally needed (4).
Second, easy recovery from TEA is surely helpful to avoid a postoperative myasthenic crisis leading to reintubation and mechanical ventilation. On the other hand, crisis occurrence depends also on the degree of preoperative myasthenic symptoms. Awake surgery can be volatile, particularly in patients with severe respiratory distress or bulbar palsy prior to the operation.
Since non-intubated surgery cannot be maintained under a completely ‘awake’ condition, some sedative agents are typically used. They induced sedation by propofol, which seems safe for myasthenic patients. Nevertheless, it remains controversial which agents have neurologically fewer adverse effects in these patients.
Thymus, thymoma, and MG
Weakness accompanying MG is mediated by autoantibodies that deplete nicotinic acetylcholine receptors (AChRs) from voluntary muscles so that they cannot be optimally triggered (5). A very puzzling but interesting characteristic of MG is that many affected patients have an abnormality, a hyperplastic thymus, as well as a thymoma. Studies show that 16.3% of thymoma patients have clinical diagnosis of MG, and all of them have the anti-AChR antibody in serum. Furthermore, 26% of patients with a thymoma and without muscle weakness were also anti-AChR positive (6). Thus, the very high rate of association of thymoma with the anti-AChR antibody strongly suggests that they have a cause—effect relationship (7).
Younger-onset MG patients without a thymoma usually have a hyperplastic thymus with numerous germinal centers (GCs) in the medulla and the appearance of a lymph node (5). The thymus is the only extra-muscular site where AChR is normally present and seems the likeliest site of autoimmunization in cases of MG. The GCs provide an environment where AChR-specific B cells undergo proliferation and increase the affinity of the antibody they produce to AChR via a process called affinity maturation (8). Isolated AChR subunits in some medullary thymic epithelial cells are believed to induce self-tolerance in developing T cells under normal conditions, but could instead autoimmunize them under abnormal conditions (9). Rare muscle-like myoid cells in the medulla express intact AChR and have been deeply implicated in formation of the characteristic GCs in ‘hyperplastic’ MG thymi, where autoantibody response diversifies to recognize the native AChR conformation (8,9) and continual generation of AChR antibody-producing plasma cells occurs (8,10) (Figure 1). The rationale of a thymectomy for non-thymomatous MG is to interrupt these processes and prevent dissemination of the response to the periphery (11).
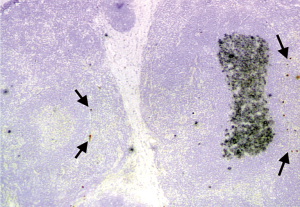
Although thymomas are rare, they are the most frequent tumor occurring in the mediastinum and originate from thymic epithelial cells arising within the thymus. They are relatively benign, tend to grow slowly, and seldom metastasize. Interestingly, they behave like immunological functioning tumors. We have been trying to identify the missing link between the thymoma and MG.
Most thymomas (especially WHO type AB and B) are associated with a large number of T cells with an immature phenotype, which do not have malignant potential. These immature T cells in the thymoma express both CD4 and CD8 on their surfaces, thus are double-positive T cells (12). This phenotype is also seen in T cells in the cortex of a normal thymus, though not in the periphery (peripheral blood, lymph node, spleen). Therefore, a thymoma has a capability to induce immature T cells, a function of cortical epithelial cells. In contrast, the function of medullary epithelial cells, which are the negative selection of developing T cells, shows a deficit in a thymoma. It is most likely that a thymoma is unable to fully perform negative selection on developing T cells and to delete autoreactive T cells such as anti-AChR antibodies (7). GCs have been seen in myasthenic patients with a thymoma, though they were identified in the adjacent thymus outside the thymoma rather than in the tumor itself (13). Remarkably, cells in the adjacent thymic remnants spontaneously produce anti-AChR antibodies, just as in hyperplastic thymi in younger-onset MG patients without a thymoma (14) (Figure 2).
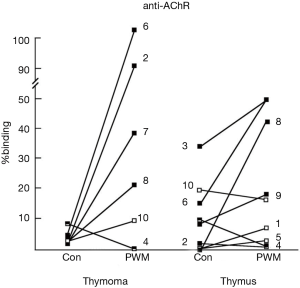
Thus, the final goal of surgery for a thymoma patient with MG is to resect as much thymic tissue in the anterior mediastinum as possible, including the tumor.
Uniportal surgery for a thymectomy procedure
Results of the MGTX trial presented in 2016 showed the benefits of a thymectomy for generalized MG (15). The surgical procedure adopted was an ‘extended’ thymectomy via a sternotomy, which anatomically defined the extent of resection of the thymus and fatty tissue (16). When we initially introduced a thoracoscopic extended thymectomy procedure at our institution, a bilateral thoracoscopic approach, anterior chest wall lifting, and additional cervical incision for resection of the upper pole were combined in order to ensure the extent of resection and safety, while remaining compatible with the concept of less invasiveness (17). The prognosis of patients who underwent our video-assisted thoracoscopic extended thymectomy (VATET) was shown to be comparable to those who received a transsternal approach in a historical control study (18). We then carefully simplified our procedure based on accumulated experience (19).
Surgeons should also take note of the oncological aspect of a thymoma, as the most frequent type of tumor relapse after a complete resection is pleural dissemination (20). We reported regarding the risk of postoperative pleural recurrence in cases with a thymoma greater than 5 cm or with a large cystic portion (21), with injury of the capsule during the operation the primary factor. Therefore, delicate manipulation is extremely important to avoid tumor relapse caused by operative factors.
Then, an important question is whether uniportal video-assisted thoracic surgery (VATS) is feasible for a thymectomy. A single port access offers the potential advantage of minimizing chest wall damage leading to less pain, early recovery, and better cosmesis. In contrast, some of the limitations encountered with a single access thus far include fencing of instruments, difficulty with suitable staple insertion, and especially urgent management in cases of unexpected trouble such as major bleeding. In most reports regarding uniportal VATS, usually employed for major or minor lung resection, a lateral decubitus position is favorable. However, they also reported that the patients were in a 30° semilateral position when undergoing a uniportal VATS thymectomy (2). It seems that they utilized this position to mobilize the ipsilateral lung posteriorly. I am concerned about maneuverability when using a 30° semilateral position through a single access. Which approach do they choose, a thoracotomy or sternotomy, in cases with critical events such as pleural adhesion or invasion to vessels? Improvements in maneuverability by development of more flexible instruments might make this method superior to multi-portal VATS.
Therefore, inclusion criteria are important keys for performing a uniportal VATS thymectomy safely and comfortably. They chose severity of myasthenia, body mass index, and stage I thymoma with a maximal diameter <5 cm, which are reasonable parameters. Theoretically, it seems that a longer operation time increases the risk of tumor injury. In our series, operation time was extended in cases with a tumor greater than 5 cm in diameter and careful handling is needed for lesions with a large cystic portion (21). Cases with invasion to adjacent organs should be excluded with computed tomography (CT) or magnetic resonance imaging (MRI). Since careful attention must be taken in accessing around the left brachiocephalic vein, 3D imaging of the thymic veins is helpful for navigation during the operation and increases the safety of any VATS thymectomy procedures (22).
Alternatively, we and others have reported uniportal VATS by use of an infrasternal approach in a supine position, in which pleural adhesion does not impair an endoscopic approach into the anterior mediastinum (23,24). Also, conversion to a sternotomy can easily be accomplished even in cases with pleural adhesion or some critical events. An infrasternal approach is expected to be more comfortable in cases of uniportal surgery for an anterior mediastinal lesion.
It is quite understandable that Pompeo’s group was able to attain combination NITS with uniportal thoracic surgery while aiming for a less invasive thymectomy. Their procedure is certainly evolutionary in terms of both a minimally invasive approach and anesthesia for an MG-associated thymoma, and they are one of the most experienced groups dealing with not only VATS thymectomy but also uniportal thoracic surgery. Since MG and thymoma cases are not frequently encountered in most institutions, this report cannot suggest that uniportal VATS thymectomy should be widely employed. Above all, analysis of the long-term prognosis regarding both myasthenia and thymoma is warranted, and is highly anticipated.
Continued progress in minimally invasive surgery in this field will certainly be achieved. Nevertheless, the concept of a thymectomy for treatment of MG patients with a thymoma is the same as that with open surgery. For development of effective surgical procedures, achievement of a proper balance between less invasiveness and radical capability with safety will always be required, leading to benefits for patients.
Acknowledgments
The author thanks Professor Nick Willcox, Oxford University, for critical advice and encouragement.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by Section Editor Yun Che (Cancer Hospital of Chinese Academy of Medical Sciences, Peking Union Medical College, Beijing, China).
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med.2017.10.04). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mineo TC. Epidural anesthesia in awake thoracic surgery. Eur J Cardiothorac Surg 2007;32:13-9. [Crossref] [PubMed]
- Pompeo E, Dauri M, Massa R, et al. Minimalist thoracoscopic resection of thymoma associated with myasthenia gravis. J Thorac Cardiovasc Surg 2017;154:1463-5. [Crossref] [PubMed]
- Tsunezuka Y, Oda M, Matsumoto I, et al. Extended thymectomy in patients with myasthenia gravis with high thoracic epidural anesthesia alone. World J Surg 2004;28:962-5. [Crossref] [PubMed]
- Matsumoto I, Oda M, Watanabe G. Awake endoscopic thymectomy via an infrasternal approach using sternal lifting. Thorac Cardiovasc Surg 2008;56:311-3. [Crossref] [PubMed]
- Leite MI, Jones M, Strobel P, et al. Myasthenia gravis thymus: complement vulnerability of epithelial and myoid cells, complement attack on them, and correlations with autoantibody status. Am J Pathol 2007;171:893-905. [Crossref] [PubMed]
- Fujii Y. The thymus, thymoma and myasthenia gravis. Surg Today 2013;43:461-6. [Crossref] [PubMed]
- Okumura M, Shiono H, Minami M, et al. Clinical and pathological aspects of thymic epithelial tumors. Gen Thorac Cardiovasc Surg 2008;56:10-16. [Crossref] [PubMed]
- Sims GP, Shiono H, Willcox N, et al. Somatic hypermutation and selection of B cells in thymic germinal centers responding to acetylcholine receptor in myasthenia gravis. J Immunol 2001;167:1935-44. [Crossref] [PubMed]
- Shiono H, Roxanis I, Zhang W, et al. Scenarios for autoimmunization of T and B cells in myasthenia gravis. Ann N Y Acad Sci 2003;998:237-56. [Crossref] [PubMed]
- Willcox HN, Newsom-Davis J, Calder LR. Greatly increased autoantibody production in myasthenia gravis by thymocyte suspensions prepared with proteolytic enzymes. Clin Exp Immunol 1983;54:378-86. [PubMed]
- Okumura M, Inoue M, Shiono H, et al. Biological implications of thymectomy for myasthenia gravis. Surg Today 2010;40:102-7. [Crossref] [PubMed]
- Okumura M, Miyoshi S, Fujii Y, et al. Clinical and functional significance of WHO classification on human thymic epithelial neoplasms: a study of 146 consecutive tumors. Am J Surg Pathol 2001;25:103-10. [Crossref] [PubMed]
- Fujii Y, Monden Y, Nakahara K, et al. Antibody to acetylcholine receptor in myasthenia gravis: production by lymphocytes from thymus or thymoma. Neurology 1984;34:1182-6. [Crossref] [PubMed]
- Shiono H, Wong YL, Matthews I, et al. Spontaneous production of anti-IFN-alpha and anti-IL-12 autoantibodies by thymoma cells from myasthenia gravis patients suggests autoimmunization in the tumor. Int Immunol 2003;15:903-13. [Crossref] [PubMed]
- Wolfe GI, Kaminski HJ, Aban IB, et al. Randomized trial of thymectomy in myasthenia gravis. N Engl J Med 2016;375:511-22. [Crossref] [PubMed]
- Masaoka A, Monden Y. Comparison of the results of transsternal simple, transcervical simple, and extended thymectomy. Ann N Y Acad Sci 1981;377:755-65. [Crossref] [PubMed]
- Shigemura N, Shiono H, Inoue M, et al. Inclusion of the transcervical approach in video-assisted thoracoscopic extended thymectomy (VATET) for myasthenia gravis: a prospective trial. Surg Endosc 2006;20:1614-8. [Crossref] [PubMed]
- Shiono H, Kadota Y, Hayashi A, Okumura M. Comparison of outcomes after extended thymectomy for myasthenia gravis: bilateral thoracoscopic approach versus sternotomy. Surg Laparosc Endosc Percutan Tech 2009;19:424-7. [Crossref] [PubMed]
- Nakagiri T, Inoue M, Shintani Y. Improved procedures and comparative results for video-assisted thoracoscopic extended thymectomy for myasthenia gravis. Surg Endosc 2015;29:2859-65. [Crossref] [PubMed]
- Utsumi T, Shiono H, Matsumura A, et al. Stage III thymoma: relationship of local invasion to recurrence. J Thorac Cardiovasc Surg 2008;136:1481-5. [Crossref] [PubMed]
- Kimura T, Inoue M, Shiono H, et al. The oncological feasibility and limitations of video-assisted thoracoscopic thymectomy for early-stage thymomas. Eur J Cardiothorac Surg 2013;44:e214-8. [Crossref] [PubMed]
- Shiono H, Inoue A, Tomiyama N, et al. Safer video-assisted thoracoscopic thymectomy after location of thymic veins with multidetector computed tomography. Surg Endosc 2006;20:1419-22. [Crossref] [PubMed]
- Shiono H, Nishiki K, Ikeda M. Single-incision surgery with SILS port for anterior mediastinal lesions: initial experience. Surg Laparosc Endosc Percutan Tech 2011;21:e225-7. [Crossref] [PubMed]
- Suda T, Sugimura H, Tochii D. Single-port thymectomy through an infrasternal approach. Ann Thorac Surg 2012;93:334-6. [Crossref] [PubMed]
Cite this article as: Shiono H. Balance between less invasiveness and radical capability with safety. Mediastinum 2017;1:15.


