
Induction chemoradiotherapy for unresectable thymic tumors
Introduction
The majority of thymomas are discovered at an early stage, Masaoka-Koga stage I or II or T1a-b2N0M0 (proposed 8th edition staging of thymic tumors) (1), where surgical resection remains the mainstay of curative treatment. Approximately 1/3rd of patients will present with locally advanced thymoma, namely stage III disease, where a multimodality approach has become the care standard. Induction chemotherapy has been widely adopted for locally advanced disease despite the lack of randomized clinical data (2,3). Combination chemotherapy + radiation as an induction strategy has appeal since the biologic interaction may potentiate treatment effect and enhance complete resection. Preoperative radiation allows for more precise planning compared to the post-operative treatment volume delivered to the bed of the resected tumor. Proponents of induction chemoradiotherapy hypothesized that there would be less chance for pleural seeding during surgical resection and fewer local recurrences.
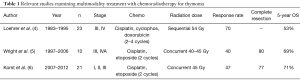
Evaluation of induction chemotherapy combined with radiation for locally advanced or “unresectable” thymic neoplasm is poorly studied. One of the earliest prospective multicenter trials that examined the efficacy of chemotherapy followed by radiation for limited stage unresectable thymoma was performed in a cooperative group trial and published in 1997 (4). This study analyzed 23 patients accrued from 1983–1995 with thymoma and thymic carcinoma [Masaoka stage III (n=22), stage IVA (n=1)] who received 2–4 cycles of chemotherapy (cisplatin, doxorubicin, cyclophosphamide) followed sequentially by 54 Gy of intensity-modulated radiation therapy (IMRT) to the primary tumor and regional lymph nodes. Chemotherapy response alone was 70% and the addition of radiation increased the complete or partial response rate in 5/23 patients. No patient underwent surgical resection. A local failure rate of 65% was observed and results showed that patients treated with 4 cycles of chemotherapy had improved survival compared to 2 cycles. The authors reported a 53% 5-year overall survival (Table 1).
Full table
Since induction chemotherapy given concurrently with radiation has been successfully used to improve complete resection in advanced thoracic cancers such as superior sulcus tumors (7) or locally advanced distal esophageal cancers (8), our institution employed a strategy of 2 cycles of chemotherapy (cisplatin, etoposide) given in combination with 40–45 Gy IMRT to the primary tumor followed by surgical resection. Wright and colleagues reported on this retrospective series of 10 patients accumulated from 1997–2006 (5). Most patients (80%) harbored tumors >8 cm and the study included 7 patients with Masaoka stage III, and 3 patients with stage IVA disease (see Table 1). Only one patient had a thymic carcinoma. The radiographic response rate was only 40%; likely related to a reduced dose intensity of chemotherapy. All patients underwent surgery 4–8 weeks after induction where complete resection (R0) was achieved in 8 (80%) patients. No patient exhibited a complete pathologic response; however, 4 patients had near pathologic complete response. Of note, PET scan did not appear to correlate with radiographic response or pathologic response. The authors reported a 69% 5-year overall survival with a median follow-up of 41 months.
The published results from Wright et al., spawned a prospective, multi-institutional phase II trial of induction treatment with 2 cycles of cisplatin/etoposide concurrently with 45 Gy IMRT (6). The study enrolled 21 patients from four institutions with specific inclusion criteria and included 14 patients with thymoma and 7 patients with thymic carcinoma (see Table 1). A radiographic response was noted in 47% of patients (best seen in thymic carcinoma). Complete resection was achieved in 77% of patients. Surprisingly again, no complete pathologic responses were encountered. In contrast, induction chemotherapy alone has been associated with a 10–15% complete response rate in reported series (9-12). It remains to be seen if an excellent pathologic response translates into a more favorable prognosis. Five patients had <10% viable tumor (4 of these were thymic carcinomas). Nine patients (41%) experienced a grade III/IV toxicity and there were 2 postoperative deaths. The authors reported 71% 5-year overall survival. Although there was a high rate of complete surgical resection, superiority of induction chemoradiotherapy over induction chemotherapy alone was not established with this trial.
Other than tumor stage, R0 resection remains the most consistent prognostic factor in published series. Although, combination induction chemoradiotherapy for thymic tumors appears to increase the rate of complete resection, there is clearly more associated treatment toxicity and the surgical field becomes more difficult to navigate by virtue of radiation fibrosis. Locally advanced thymomas that appear difficult to resect should be considered for induction chemotherapy to maximize the chance of a complete resection. If a surgeon is already committed where complete resection cannot be safely achieved, it makes sense to debulk as much tumor as possible balancing the risks to adjacent structures and postoperative complications with the benefit of reducing tumor bulk in an otherwise indolent tumor. Debulking has been associated with improved overall survival in a meta-analysis comparing incomplete resection to observation in “unresectable” thymoma (13). The surgeon should then leave clips behind to facilitate directed postoperative radiation therapy.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors Mirella Marino and Brett W. Carter for the series “Dedicated to the 8th International Thymic Malignancy Interest Group Annual Meeting (ITMIG 2017)” published in Mediastinum. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med.2017.10.05). The series “Dedicated to the 8th International Thymic Malignancy Interest Group Annual Meeting (ITMIG 2017)” was commissioned by the editorial office without any funding or sponsorship. This study has been presented at 8th International Thymic Malignancy Interest Group Annual Meeting (ITMIG 2017). The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Detterbeck FC, Stratton K, Giroux D, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposal for an evidence-based stage classification system for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol 2014;9:S65-72. [Crossref] [PubMed]
- Lemma GL, Lee JW, Aisner SC, et al. Phase II study of carboplatin and paclitaxel in advanced thymoma and thymic carcinoma. J Clin Oncol 2011;29:2060-5. [Crossref] [PubMed]
- Ahmad U, Huang J. Induction Therapy for Thymoma. Thorac Surg Clin 2016;26:325-32. [Crossref] [PubMed]
- Loehrer PJ Sr, Kim K, Aisner SC, et al. Cisplatin plus doxorubicin plus cyclophosphamide in metastatic or recurrent thymoma: final results of an intergroup trial. The Eastern Cooperative Oncology Group, Southwest Oncology Group, and Southeastern Cancer Study Group. J Clin Oncol 1994;12:1164-8. [Crossref] [PubMed]
- Wright CD, Choi NC, Wain JC, et al. Induction chemoradiotherapy followed by resection for locally advanced Masaoka stage III and IVA thymic tumors. Ann Thorac Surg 2008;85:385-9. [Crossref] [PubMed]
- Korst RJ, Bezjak A, Blackmon S, et al. Neoadjuvant chemoradiotherapy for locally advanced thymic tumors: a phase II, multi-institutional clinical trial. J Thorac Cardiovasc Surg 2014;147:36-44, 46.e1.
- Rusch VW, Giroux DJ, Kraut MJ, et al. Induction chemoradiation and surgical resection for superior sulcus non-small-cell lung carcinomas: long-term results of Southwest Oncology Group Trial 9416 (Intergroup Trial 0160). J Clin Oncol 2007;25:313-8. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Lucchi M, Melfi F, Dini P, et al. Neoadjuvant chemotherapy for stage III and IVA thymomas: a single-institution experience with a long follow-up. J Thorac Oncol 2006;1:308-13. [Crossref] [PubMed]
- Kunitoh H, Tamura T, Shibata T, et al. A phase II trial of dose-dense chemotherapy, followed by surgical resection and/or thoracic radiotherapy, in locally advanced thymoma: report of a Japan Clinical Oncology Group trial (JCOG 9606). Br J Cancer 2010;103:6-11. [Crossref] [PubMed]
- Kim ES, Putnam JB, Komaki R, et al. Phase II study of a multidisciplinary approach with induction chemotherapy, followed by surgical resection, radiation therapy, and consolidation chemotherapy for unresectable malignant thymomas: final report. Lung Cancer 2004;44:369-79. [Crossref] [PubMed]
- Hamaji M, Ali SO, Burt BM. A meta-analysis of induction therapy for advanced thymic epithelial tumors. Ann Thorac Surg 2015;99:1848-56. [Crossref] [PubMed]
- Hamaji M, Kojima F, Omasa M, et al. A meta-analysis of debulking surgery versus surgical biopsy for unresectable thymoma. Eur J Cardiothorac Surg 2015;47:602-7. [Crossref] [PubMed]
Cite this article as: Lanuti M. Induction chemoradiotherapy for unresectable thymic tumors. Mediastinum 2017;1:13.


