
Overview of the pathology of thymic neuroendocrine tumors
Definition
In the WHO classification of tumors of the lung, pleura, thymus and heart published in March 2015 (1), lung and thymic neuroendocrine tumors (NETs) have been incorporated into a new category of neuroendocrine neoplasms, and have been similarly classified into low-grade typical carcinoids (TCs), intermediate-grade atypical carcinoids (ACs) and high-grade poorly differentiated neuroendocrine carcinomas (HGNECs) of the large and small cell types (LCNEC and SCLC). Combined thymoma or thymic carcinoma and NET (usually high-grade NE carcinomas) rarely occur. It may be impossible to assess the exact origin of these tumors in the presence of large and centrally located NETs, infiltrating the mediastinal pleura and lung on one side and the mediastinal tissues (including thymus) on the other. In fact, no single marker of NET cells is absolutely specific to lung versus thymic derivation, or even versus mediastinal metastases of gastroenteropancreatic NETs (except for some hormonally active tumors).
Diagnostic criteria
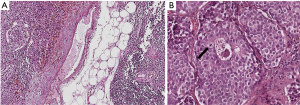
At variance with lung carcinoids, primary thymic carcinoids are mostly ACs, being TCs extremely rare. As for their pulmonary counterpart, pure morphological criteria are the only appropriate tools for identifying the different histotypes, combining architectural (organoid versus diffuse growth) and cytological features (cell size, atypia, mitotic count, necrosis) (Figure 1A,B). Apart from the two forms of carcinoids, LCNEC has been reclassified from the group of large cell carcinomas to the new group of NE neoplasms and is defined by large atypical cells, usually arranged in nests and trabecula, possibly forming rosette-like structures, and with irregular nuclei with granular chromatin and conspicuous nucleoli (2). Necrosis is often more extensive and mitotic count exceeds 10 per 10 high power fields even in cases that maintain a well differentiated/organoid morphology. Paraneoplastic symptoms due to abnormal hormones production, usually ACTH, are frequent and have been reported also in thymic LCNEC (3). Combined cases of thymoma or thymic carcinoma and NET are rarely observed in the thymus and are characterized by the coexistence of a NE component along with one of thymic tumor types. Since neuroendocrine differentiation in both thymomas and thymic carcinomas is possible (4,5), diagnosis of a coexisting neoplasm requires the identification of morphological features of both tumors (6-8). The pathogenesis of such combined thymic tumors is not fully understood, although genetic data from other locations (e.g., lung or pancreas) mostly showed a similar genetic profile in the two components, suggesting a divergent differentiation of a single neoplasia.
Most diagnostic difficulties derive from small biopsies or cytological samples, in which the scant amount of tumor cells may hamper a correct interpretation. While the definition of the NE nature may not be difficult, thanks to the demonstration of specific NE markers like synaptophysin and chromogranin, and the lack of high molecular weight cytokeratin expression in all NE tumors, regardless of their location, the definition of the histotype is less reliable in such materials, especially the separation of low-grade carcinoids from high-grade NE carcinomas. The data mentioned above, together with our clinical experience, suggest that the most useful diagnostic marker is Ki67 (as reported in the lung) (9). This marker maintains its nuclear distribution even in tumor areas with crushing artifacts, as commonly observed in biopsies, thus allowing to distinguish carcinoids from HGNEC.
There is no specific grading system for thymic neuroendocrine neoplasms and the prognostic value of lung NETs classification criteria in thymic lesions has not been demonstrated yet.
The literature data indicate that, although the evaluation of Ki67 proliferation index has not been officially incorporated in the WHO criteria for thymic NETs (nor for pulmonary NETs), it is a reliable and useful tool to predict their behavior. In fact, it has been proposed as an additional parameter (combined with the two “official” tools, i.e., mitotic count and necrosis) for grading lung NETs (10). Probably due to their rarity, a similar study for thymic NET grading has not been performed yet. Nevertheless, the proposed cutoffs of 4 and 25% for taking low-intermediate-high grade lung NETs apart, also seem to be applicable to thymic NE neoplasms.
Genetics
Although a large number of genetic studies on pulmonary NETs have recently been published, including NGS data (11,12), thymic NETs have been rarely addressed. In carcinoids, RB1 and TP53 mutations were uncommon, whereas MEN1 mutations are known to occur in thymic NETs, along with CDKN2A alterations (13). High-grade carcinomas (LCNEC and SCLC) shared gene mutations with similar tumors of other locations (e.g., lung), including RB1, TP53, PTEN (14), as well as amplifications of MYC gene (15). In a genetic study of 73 cases from multiple institutions, 13 TCs, 40 ACs, and 20 HGNECs were investigated. Chromosomal imbalances were identified at increasing mean numbers per tumor from 0.8 in TCs to 1.1 in AC cases, up to 4.7 in HGNECs (also the percentage of aberrant cases increased from 31%, to 44% and 75% in TCs, ACs and HGNECs, respectively). The most frequently detected genetic changes in both carcinoids and HGNECs were gains at the MYC gene locus (8q24) (15). Whole exome sequencing of a series of 9 thymic NETs with ectopic ACTH secretion syndrome identified three genes (HRAS, PAK1 and MEN1) potentially involved in tumorigenesis (16).
Predictive biomarkers
Predictors of response to specific treatments (including mTOR pathway alterations, YY1 mutations and SSTR expression), as reported in pulmonary and gastroenteropancreatic NETs (17,18), have not been extensively investigated in thymic NETs, except for single case reports.
Response to treatment with receptor tyrosine kinases inhibitors (sunitinib and imatinib) has been reported in thymic NET (19,20). mTOR pathway inhibitors, such as everolimus, have shown efficacy in NET of multiple sites and seem promising also in thymic neuroendocrine neoplasms (21). Recently, expression of five potential predictive biomarkers (CD52, CD22, CD26, EG5 and IGF-1R) has been evaluated in a series of 5 thymic carcinoids identifying only rare expression of CD22 and EG5 (22).
Conclusions
Although the current classification of thymic NETs reflects tumor biology and it is prognostically relevant, accepted worldwide and familiar to clinicians, an improved homogenization or better integration of lung, thymic, gastrointestinal and pancreatic NET classifications would be highly welcome, at least in terms of terminology and histological categories.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors Mirella Marino and Brett W. Carter for the series “Dedicated to the 8th International Thymic Malignancy Interest Group Annual Meeting (ITMIG 2017)” published in Mediastinum. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med.2017.10.06). The series “Dedicated to the 8th International Thymic Malignancy Interest Group Annual Meeting (ITMIG 2017)” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Travis WD, Brambilla E, Burke A, et al. WHO classification of tumours of the lung, pleura, thymus and heart. Lyon: International Agency for Research on Cancer, 2015.
- Boubacar E, Atsame-Ebang G, Rabiou S, et al. Thymic large cell neuroendocrine carcinoma - a rare and aggressive tumor: a case report. J Med Case Rep 2017;11:155. [Crossref] [PubMed]
- Saito T, Kimoto M, Nakai S, et al. Ectopic ACTH syndrome associated with large cell neuroendocrine carcinoma of the thymus. Intern Med 2011;50:1471-5. [Crossref] [PubMed]
- Weissferdt A, Hernandez JC, Kalhor N, et al. Spindle cell thymomas: an immunohistochemical study of 30 cases. Appl Immunohistochem Mol Morphol 2011;19:329-35. [Crossref] [PubMed]
- Weissferdt A, Moran CA. Neuroendocrine Differentiation in Thymic Carcinomas: A Diagnostic Pitfall: An Immunohistochemical Analysis of 27 Cases. Am J Clin Pathol 2016;145:393-400. [Crossref] [PubMed]
- Mizuno T, Masaoka A, Hashimoto T, et al. Coexisting thymic carcinoid tumor and thymoma. Ann Thorac Surg 1990;50:650-2. [Crossref] [PubMed]
- Sensaki K, Aida S, Takagi K, et al. Coexisting undifferentiated thymic carcinoma and thymic carcinoid tumor. Respiration 1993;60:247-9. [Crossref] [PubMed]
- Miller BS, Rusinko RY, Fowler L. Synchronous thymoma and thymic carcinoid in a woman with multiple endocrine neoplasia type 1: case report and review. Endocr Pract 2008;14:713-6. [Crossref] [PubMed]
- Pelosi G, Rodriguez J, Viale G, et al. Typical and atypical pulmonary carcinoid tumor overdiagnosed as small-cell carcinoma on biopsy specimens: a major pitfall in the management of lung cancer patients. Am J Surg Pathol 2005;29:179-87. [Crossref] [PubMed]
- Rindi G, Klersy C, Inzani F, et al. Grading the neuroendocrine tumors of the lung: an evidence-based proposal. Endocr Relat Cancer 2013;21:1-16. [Crossref] [PubMed]
- Peifer M, Fernández-Cuesta L, Sos ML, et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat Genet 2012;44:1104-10. [Crossref] [PubMed]
- George J, Lim JS, Jang SJ, et al. Comprehensive genomic profiles of small cell lung cancer. Nature 2015;524:47-53. [Crossref] [PubMed]
- Wood K, Byron E, Janisch L, et al. Capecitabine and Celecoxib as a Promising Therapy for Thymic Neoplasms. Am J Clin Oncol 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Fernandez-Cuesta L, Peifer M, Lu X, et al. Cross-entity mutation analysis of lung neuroendocrine tumors sheds light into their molecular origin and identifies new therapeutic targets. AACR Annula Meeting 2014 American Association of Cancer Research. San Diego (CA), 2014:abstract #1531.
- Ströbel P, Zettl A, Shilo K, et al. Tumor genetics and survival of thymic neuroendocrine neoplasms: a multi-institutional clinicopathologic study. Genes Chromosomes Cancer 2014;53:738-49. [Crossref] [PubMed]
- Li Y, Peng Y, Jiang X, et al. Whole exome sequencing of thymic neuroendocrine tumor with ectopic ACTH syndrome. Eur J Endocrinol 2017;176:187-94. [Crossref] [PubMed]
- Zatelli MC, Fanciulli G, Malandrino P, et al. Predictive factors of response to mTOR inhibitors in neuroendocrine tumours. Endocr Relat Cancer 2016;23:R173-83. [Crossref] [PubMed]
- Volante M, Brizzi MP, Faggiano A, et al. Somatostatin receptor type 2A immunohistochemistry in neuroendocrine tumors: a proposal of scoring system correlated with somatostatin receptor scintigraphy. Mod Pathol 2007;20:1172-82. [Crossref] [PubMed]
- Dham A, Truskinovsky AM, Dudek AZ. Thymic carcinoid responds to neoadjuvant therapy with sunitinib and octreotide: a case report. J Thorac Oncol 2008;3:94-7. [Crossref] [PubMed]
- Hamada S, Masago K, Mio T, et al. Good clinical response to imatinib mesylate in atypical thymic carcinoid With KIT overexpression. J Clin Oncol 2011;29:e9-10. [Crossref] [PubMed]
- Ferolla P, Brizzi MP, Meyer T, et al. Efficacy and safety of pasireotide LAR or everolimus alone, or in combination in patients with advanced carcinoids (NET) of the lung/thymus: Results from the randomized, phase 2 LUNA study. Ann Oncol 2016;27:416O. [Crossref]
- Remon J, Abedallaa N, Taranchon-Clermont E, et al. CD52, CD22, CD26, EG5 and IGF-1R expression in thymic malignancies. Lung Cancer 2017;108:168-72. [Crossref] [PubMed]
Cite this article as: Bertero L, Metovic J, Vittone F, Cassoni P, Papotti M. Overview of the pathology of thymic neuroendocrine tumors. Mediastinum 2017;1:10.


